| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146481 | Chemical Engineering Journal | 2015 | 5 Pages |
•We prepared crown ether metal complex fluorides (CEMCFs) in water.•CEMCFs were used as fluoride source for nucleophilic fluorination reaction.•CEMCF/t-alcohol system showed excellent chemo-selectivity in the fluorination.•Phenolic TBDMS ether was cleaved selectively using CEMCF/methanol.
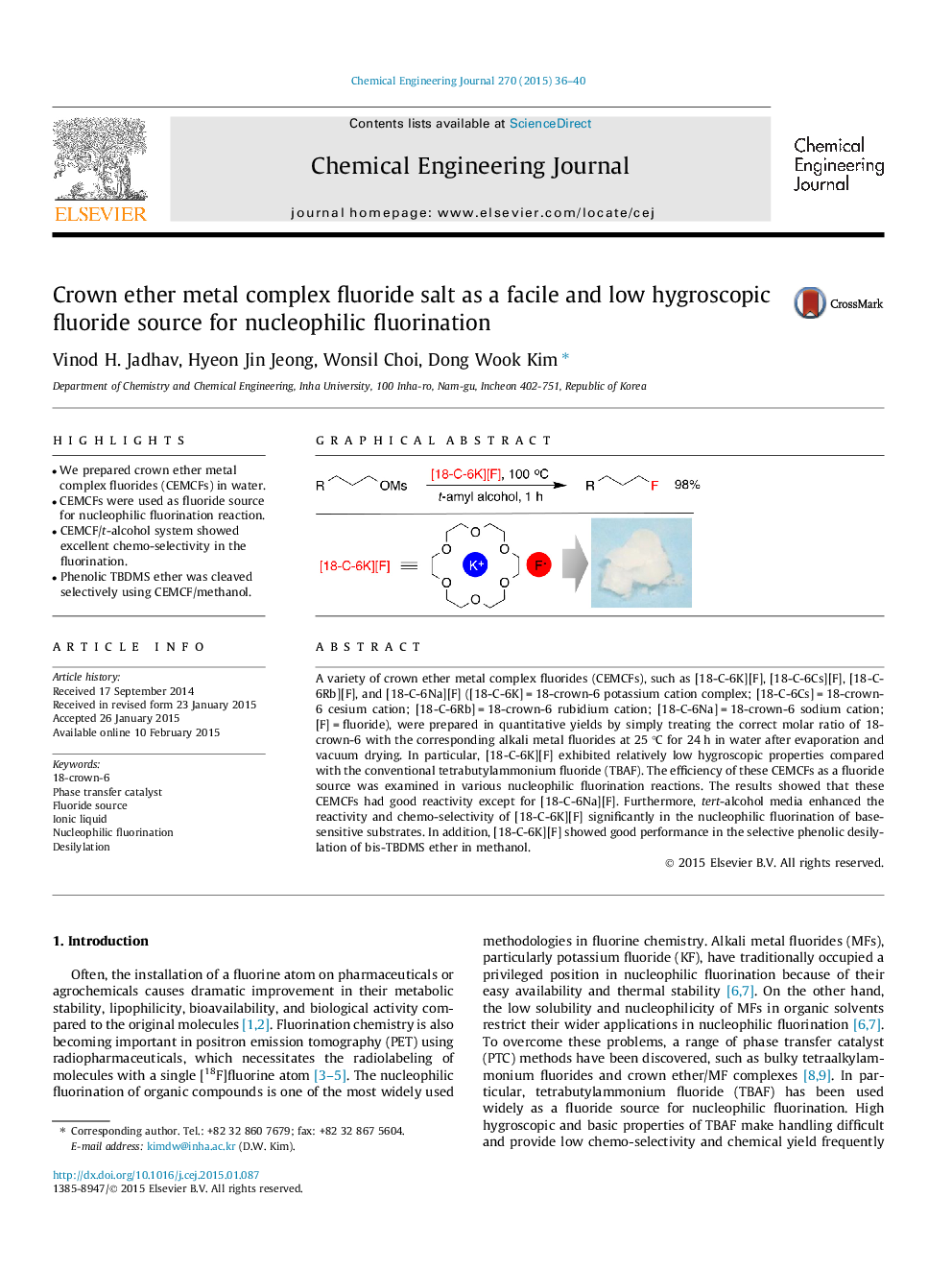
A variety of crown ether metal complex fluorides (CEMCFs), such as [18-C-6K][F], [18-C-6Cs][F], [18-C-6Rb][F], and [18-C-6Na][F] ([18-C-6K] = 18-crown-6 potassium cation complex; [18-C-6Cs] = 18-crown-6 cesium cation; [18-C-6Rb] = 18-crown-6 rubidium cation; [18-C-6Na] = 18-crown-6 sodium cation; [F] = fluoride), were prepared in quantitative yields by simply treating the correct molar ratio of 18-crown-6 with the corresponding alkali metal fluorides at 25 °C for 24 h in water after evaporation and vacuum drying. In particular, [18-C-6K][F] exhibited relatively low hygroscopic properties compared with the conventional tetrabutylammonium fluoride (TBAF). The efficiency of these CEMCFs as a fluoride source was examined in various nucleophilic fluorination reactions. The results showed that these CEMCFs had good reactivity except for [18-C-6Na][F]. Furthermore, tert-alcohol media enhanced the reactivity and chemo-selectivity of [18-C-6K][F] significantly in the nucleophilic fluorination of base-sensitive substrates. In addition, [18-C-6K][F] showed good performance in the selective phenolic desilylation of bis-TBDMS ether in methanol.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide