| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146506 | Chemical Engineering Journal | 2015 | 11 Pages |
•The cross-linked tetrapolymeric anionic polyelectrolyte (CAPE) was synthesized.•The sorption activity of CAPE was evaluated for the adsorption of chromium (III).•Adsorption process fitted the pseudo-second order kinetic model well.•The CAPE is of fast uptake with high capacity and reusability.
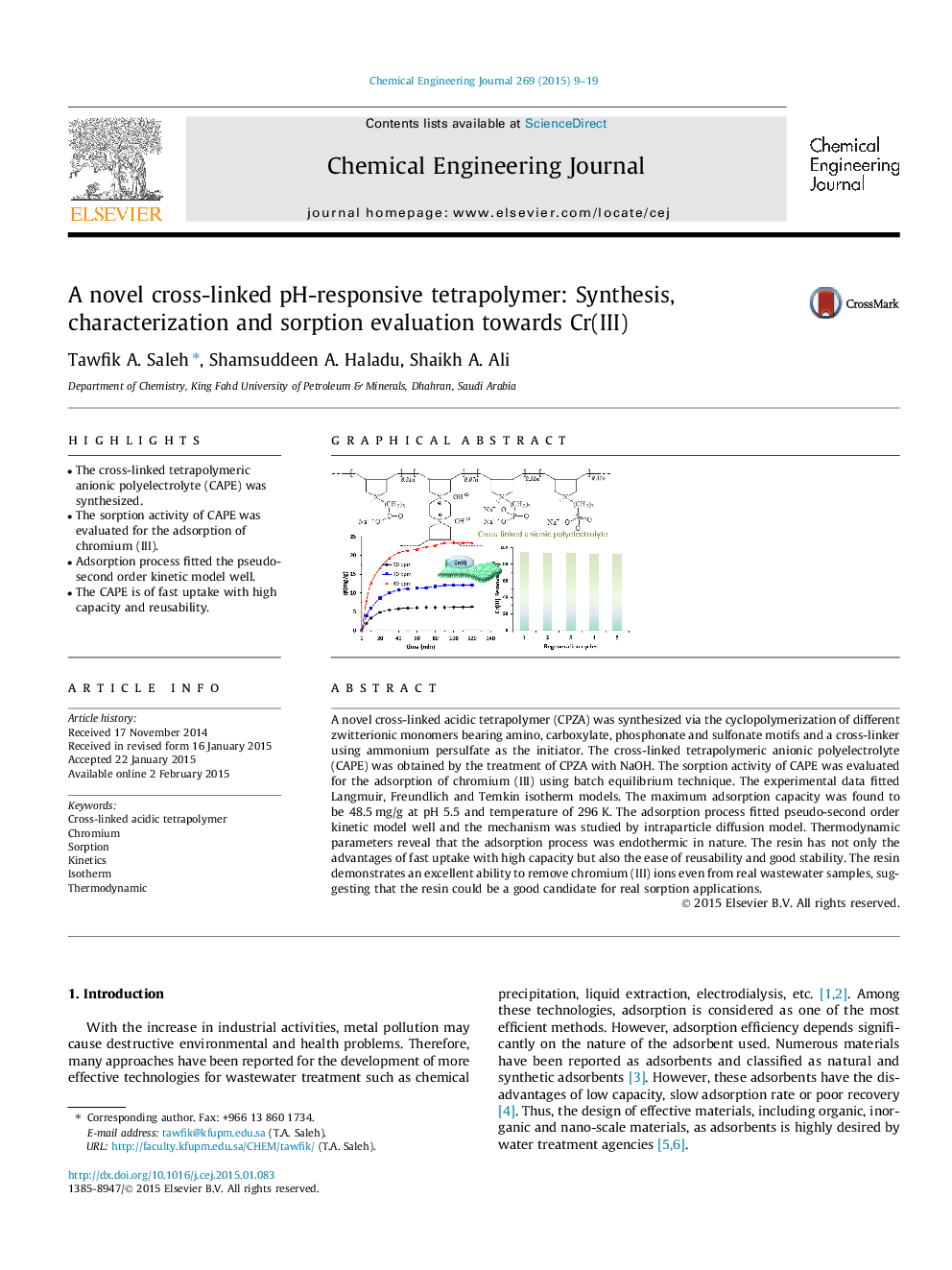
A novel cross-linked acidic tetrapolymer (CPZA) was synthesized via the cyclopolymerization of different zwitterionic monomers bearing amino, carboxylate, phosphonate and sulfonate motifs and a cross-linker using ammonium persulfate as the initiator. The cross-linked tetrapolymeric anionic polyelectrolyte (CAPE) was obtained by the treatment of CPZA with NaOH. The sorption activity of CAPE was evaluated for the adsorption of chromium (III) using batch equilibrium technique. The experimental data fitted Langmuir, Freundlich and Temkin isotherm models. The maximum adsorption capacity was found to be 48.5 mg/g at pH 5.5 and temperature of 296 K. The adsorption process fitted pseudo-second order kinetic model well and the mechanism was studied by intraparticle diffusion model. Thermodynamic parameters reveal that the adsorption process was endothermic in nature. The resin has not only the advantages of fast uptake with high capacity but also the ease of reusability and good stability. The resin demonstrates an excellent ability to remove chromium (III) ions even from real wastewater samples, suggesting that the resin could be a good candidate for real sorption applications.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide