| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146507 | Chemical Engineering Journal | 2015 | 7 Pages |
•Production of tyre char activated carbons under various experimental conditions.•Determination of effective surface area as a measure of accessible pores for certain adsorbate.•Evaluation of the phenol adsorption onto the produced activated carbons.•Determination of the significance of microporosity in phenol adsorption.•Elucidation of the phenol molecule orientation onto activated carbons using the model molecule.
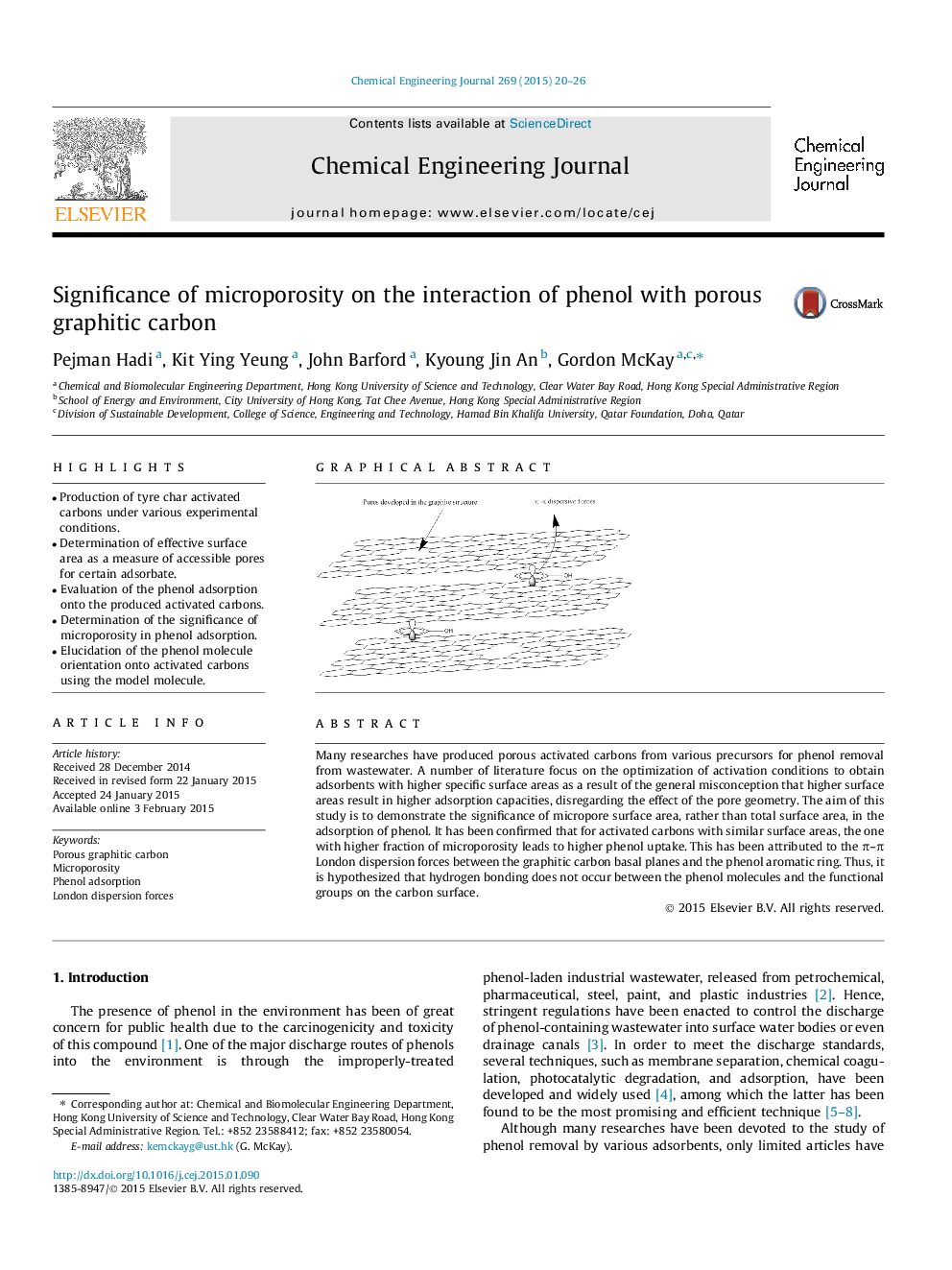
Many researches have produced porous activated carbons from various precursors for phenol removal from wastewater. A number of literature focus on the optimization of activation conditions to obtain adsorbents with higher specific surface areas as a result of the general misconception that higher surface areas result in higher adsorption capacities, disregarding the effect of the pore geometry. The aim of this study is to demonstrate the significance of micropore surface area, rather than total surface area, in the adsorption of phenol. It has been confirmed that for activated carbons with similar surface areas, the one with higher fraction of microporosity leads to higher phenol uptake. This has been attributed to the π–π London dispersion forces between the graphitic carbon basal planes and the phenol aromatic ring. Thus, it is hypothesized that hydrogen bonding does not occur between the phenol molecules and the functional groups on the carbon surface.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide