| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146509 | Chemical Engineering Journal | 2015 | 7 Pages |
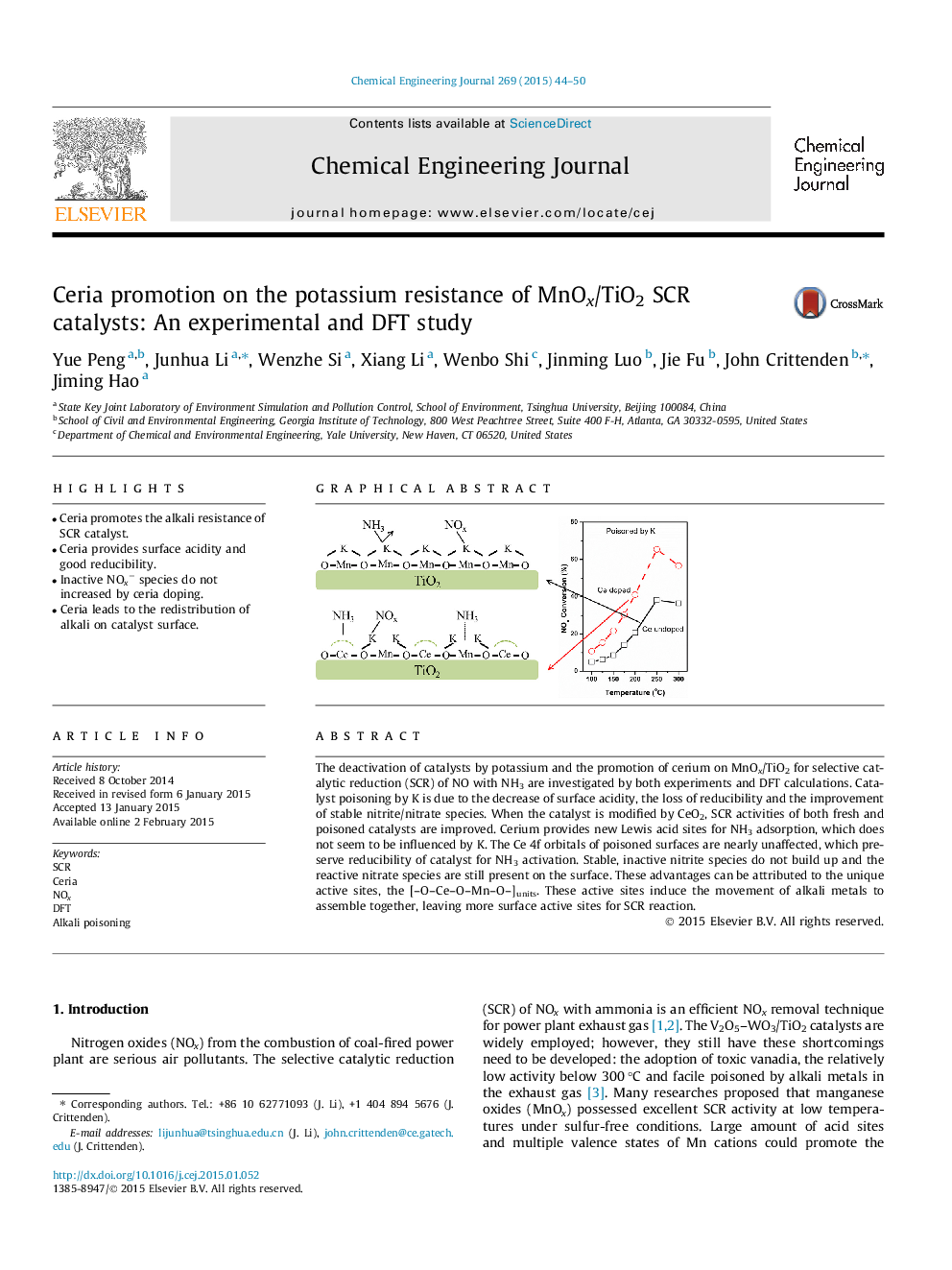
•Ceria promotes the alkali resistance of SCR catalyst.•Ceria provides surface acidity and good reducibility.•Inactive NOx− species do not increased by ceria doping.•Ceria leads to the redistribution of alkali on catalyst surface.
The deactivation of catalysts by potassium and the promotion of cerium on MnOx/TiO2 for selective catalytic reduction (SCR) of NO with NH3 are investigated by both experiments and DFT calculations. Catalyst poisoning by K is due to the decrease of surface acidity, the loss of reducibility and the improvement of stable nitrite/nitrate species. When the catalyst is modified by CeO2, SCR activities of both fresh and poisoned catalysts are improved. Cerium provides new Lewis acid sites for NH3 adsorption, which does not seem to be influenced by K. The Ce 4f orbitals of poisoned surfaces are nearly unaffected, which preserve reducibility of catalyst for NH3 activation. Stable, inactive nitrite species do not build up and the reactive nitrate species are still present on the surface. These advantages can be attributed to the unique active sites, the [–O–Ce–O–Mn–O–]units. These active sites induce the movement of alkali metals to assemble together, leaving more surface active sites for SCR reaction.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide