| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146521 | Chemical Engineering Journal | 2015 | 8 Pages |
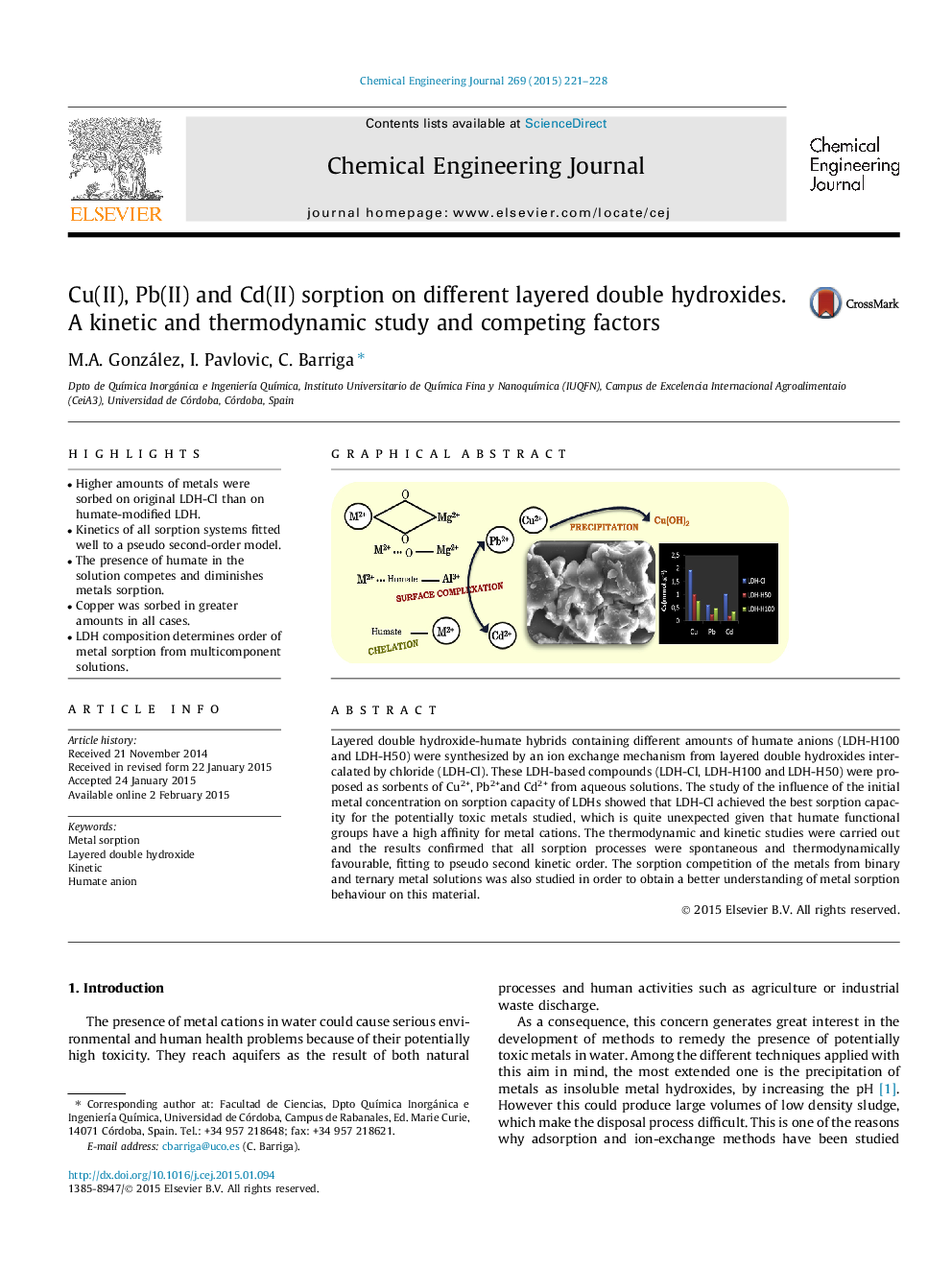
•Higher amounts of metals were sorbed on original LDH-Cl than on humate-modified LDH.•Kinetics of all sorption systems fitted well to a pseudo second-order model.•The presence of humate in the solution competes and diminishes metals sorption.•Copper was sorbed in greater amounts in all cases.•LDH composition determines order of metal sorption from multicomponent solutions.
Layered double hydroxide-humate hybrids containing different amounts of humate anions (LDH-H100 and LDH-H50) were synthesized by an ion exchange mechanism from layered double hydroxides intercalated by chloride (LDH-Cl). These LDH-based compounds (LDH-Cl, LDH-H100 and LDH-H50) were proposed as sorbents of Cu2+, Pb2+and Cd2+ from aqueous solutions. The study of the influence of the initial metal concentration on sorption capacity of LDHs showed that LDH-Cl achieved the best sorption capacity for the potentially toxic metals studied, which is quite unexpected given that humate functional groups have a high affinity for metal cations. The thermodynamic and kinetic studies were carried out and the results confirmed that all sorption processes were spontaneous and thermodynamically favourable, fitting to pseudo second kinetic order. The sorption competition of the metals from binary and ternary metal solutions was also studied in order to obtain a better understanding of metal sorption behaviour on this material.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide