| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146530 | Chemical Engineering Journal | 2015 | 8 Pages |
•Solid silica gel beads made from low cost sodium silicate precursor.•Linear trend in CO2 adsorption with increased amine loadings.•Significant CO2 adsorption at lower temperature.•Effect of surface area, pore volume, CO2 adsorption w.r.t. amine loading studied.
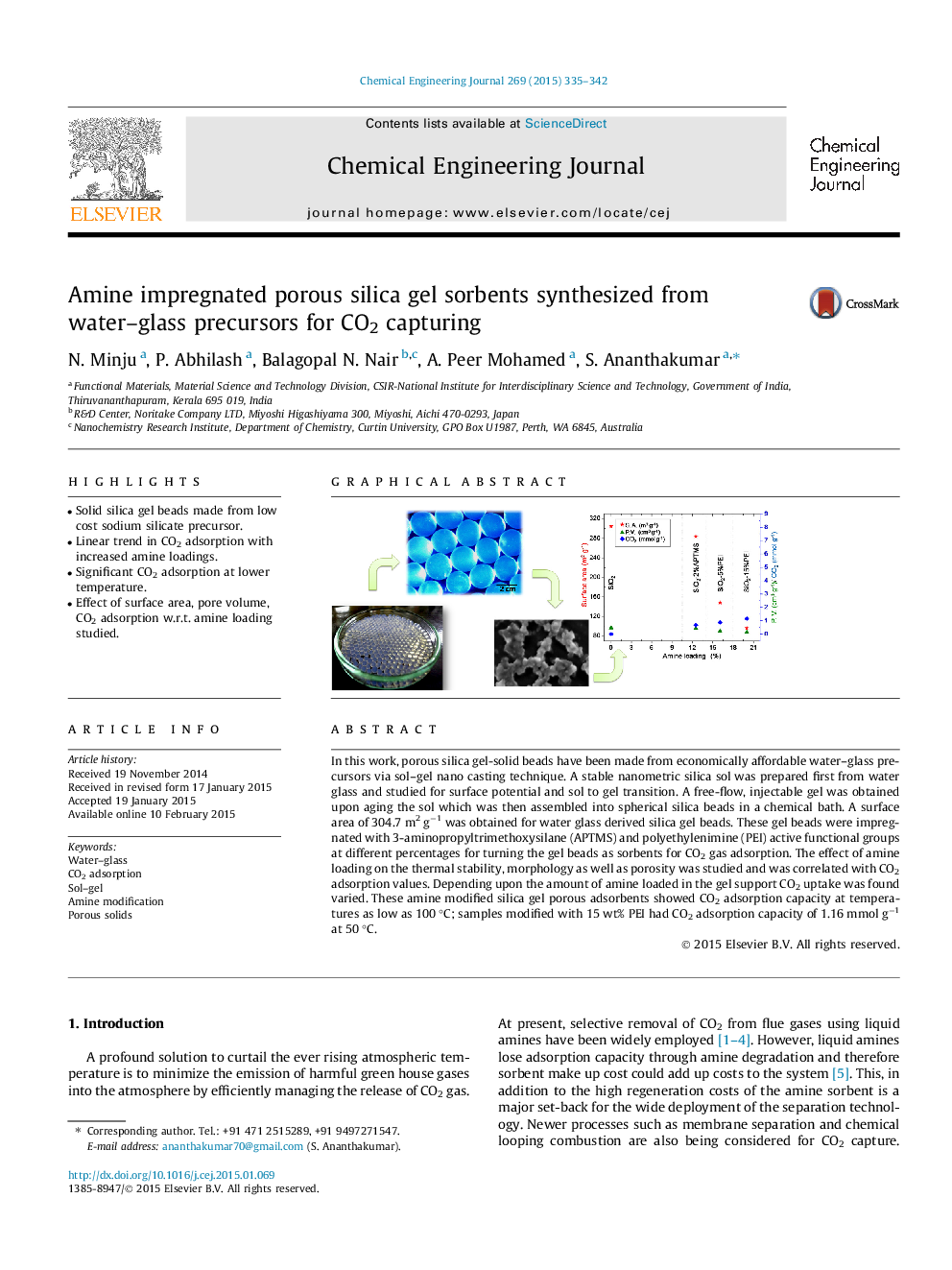
In this work, porous silica gel-solid beads have been made from economically affordable water–glass precursors via sol–gel nano casting technique. A stable nanometric silica sol was prepared first from water glass and studied for surface potential and sol to gel transition. A free-flow, injectable gel was obtained upon aging the sol which was then assembled into spherical silica beads in a chemical bath. A surface area of 304.7 m2 g−1 was obtained for water glass derived silica gel beads. These gel beads were impregnated with 3-aminopropyltrimethoxysilane (APTMS) and polyethylenimine (PEI) active functional groups at different percentages for turning the gel beads as sorbents for CO2 gas adsorption. The effect of amine loading on the thermal stability, morphology as well as porosity was studied and was correlated with CO2 adsorption values. Depending upon the amount of amine loaded in the gel support CO2 uptake was found varied. These amine modified silica gel porous adsorbents showed CO2 adsorption capacity at temperatures as low as 100 °C; samples modified with 15 wt% PEI had CO2 adsorption capacity of 1.16 mmol g−1 at 50 °C.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide