| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146539 | Chemical Engineering Journal | 2015 | 9 Pages |
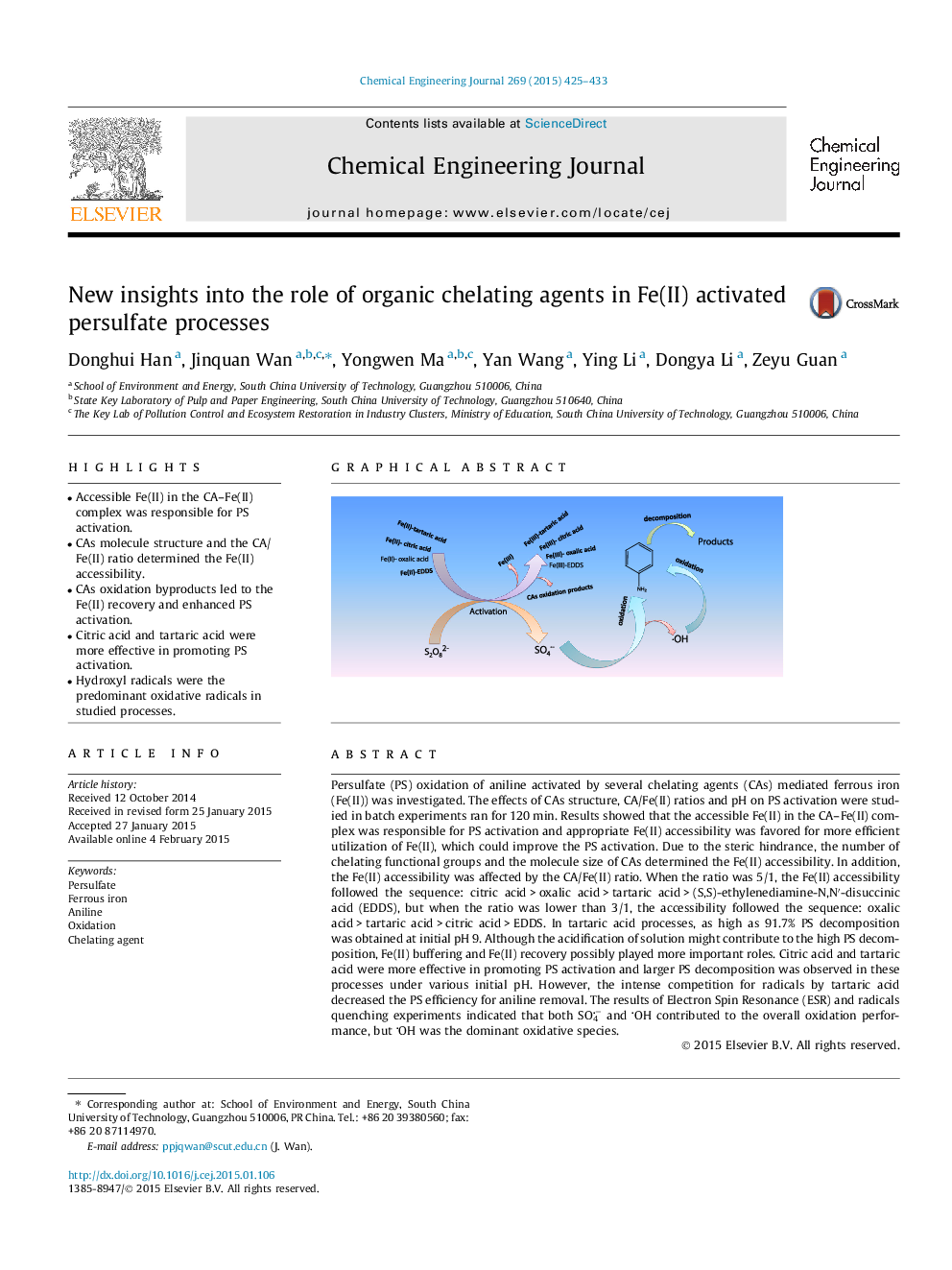
•Accessible Fe(II) in the CA–Fe(II) complex was responsible for PS activation.•CAs molecule structure and the CA/Fe(II) ratio determined the Fe(II) accessibility.•CAs oxidation byproducts led to the Fe(II) recovery and enhanced PS activation.•Citric acid and tartaric acid were more effective in promoting PS activation.•Hydroxyl radicals were the predominant oxidative radicals in studied processes.
Persulfate (PS) oxidation of aniline activated by several chelating agents (CAs) mediated ferrous iron (Fe(II)) was investigated. The effects of CAs structure, CA/Fe(II) ratios and pH on PS activation were studied in batch experiments ran for 120 min. Results showed that the accessible Fe(II) in the CA–Fe(II) complex was responsible for PS activation and appropriate Fe(II) accessibility was favored for more efficient utilization of Fe(II), which could improve the PS activation. Due to the steric hindrance, the number of chelating functional groups and the molecule size of CAs determined the Fe(II) accessibility. In addition, the Fe(II) accessibility was affected by the CA/Fe(II) ratio. When the ratio was 5/1, the Fe(II) accessibility followed the sequence: citric acid > oxalic acid > tartaric acid > (S,S)-ethylenediamine-N,N′-disuccinic acid (EDDS), but when the ratio was lower than 3/1, the accessibility followed the sequence: oxalic acid > tartaric acid > citric acid > EDDS. In tartaric acid processes, as high as 91.7% PS decomposition was obtained at initial pH 9. Although the acidification of solution might contribute to the high PS decomposition, Fe(II) buffering and Fe(II) recovery possibly played more important roles. Citric acid and tartaric acid were more effective in promoting PS activation and larger PS decomposition was observed in these processes under various initial pH. However, the intense competition for radicals by tartaric acid decreased the PS efficiency for aniline removal. The results of Electron Spin Resonance (ESR) and radicals quenching experiments indicated that both SO4− and OH contributed to the overall oxidation performance, but OH was the dominant oxidative species.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide