| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146577 | Chemical Engineering Journal | 2015 | 12 Pages |
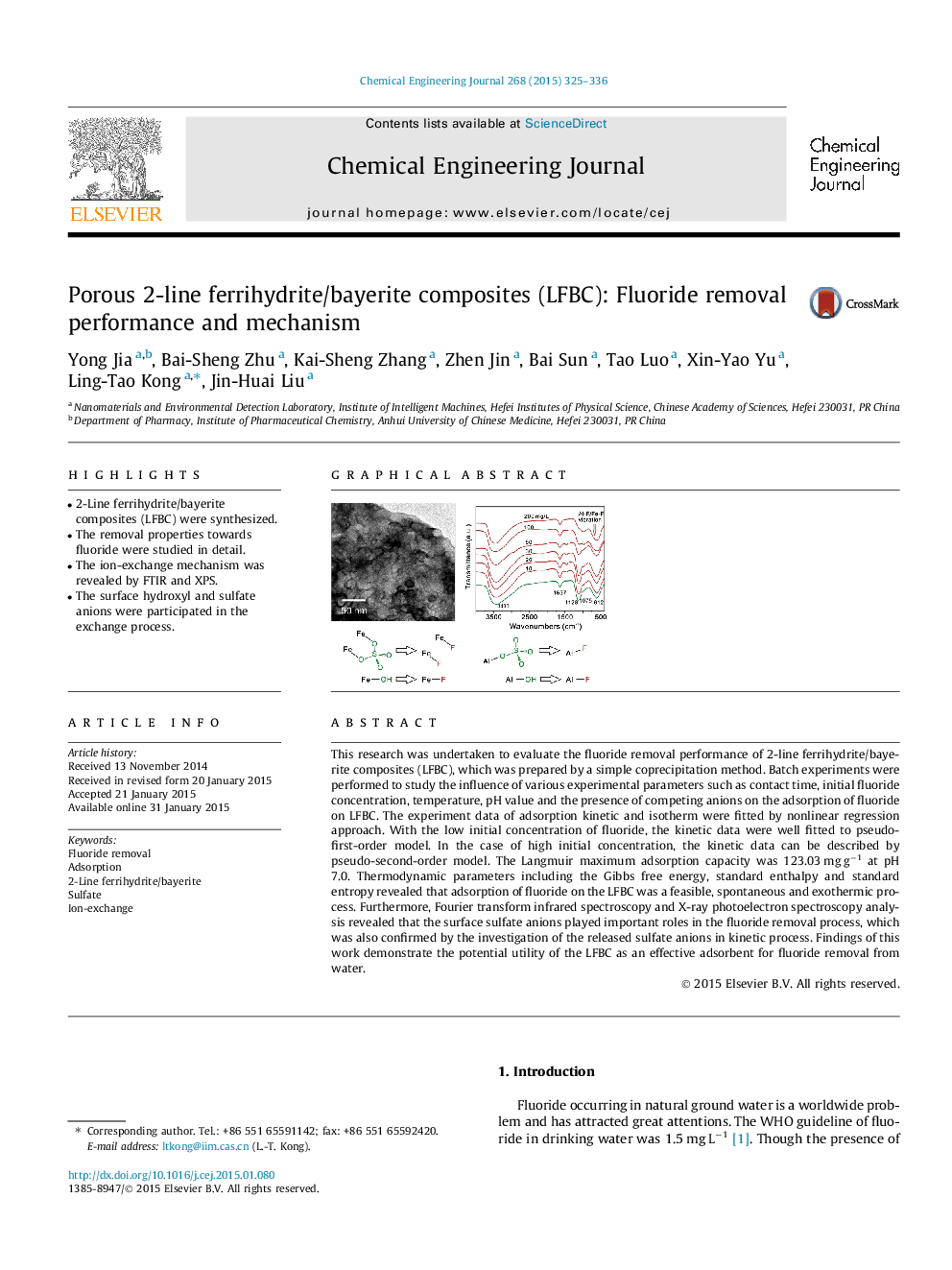
•2-Line ferrihydrite/bayerite composites (LFBC) were synthesized.•The removal properties towards fluoride were studied in detail.•The ion-exchange mechanism was revealed by FTIR and XPS.•The surface hydroxyl and sulfate anions were participated in the exchange process.
This research was undertaken to evaluate the fluoride removal performance of 2-line ferrihydrite/bayerite composites (LFBC), which was prepared by a simple coprecipitation method. Batch experiments were performed to study the influence of various experimental parameters such as contact time, initial fluoride concentration, temperature, pH value and the presence of competing anions on the adsorption of fluoride on LFBC. The experiment data of adsorption kinetic and isotherm were fitted by nonlinear regression approach. With the low initial concentration of fluoride, the kinetic data were well fitted to pseudo-first-order model. In the case of high initial concentration, the kinetic data can be described by pseudo-second-order model. The Langmuir maximum adsorption capacity was 123.03 mg g−1 at pH 7.0. Thermodynamic parameters including the Gibbs free energy, standard enthalpy and standard entropy revealed that adsorption of fluoride on the LFBC was a feasible, spontaneous and exothermic process. Furthermore, Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy analysis revealed that the surface sulfate anions played important roles in the fluoride removal process, which was also confirmed by the investigation of the released sulfate anions in kinetic process. Findings of this work demonstrate the potential utility of the LFBC as an effective adsorbent for fluoride removal from water.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide