| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146614 | Chemical Engineering Journal | 2015 | 10 Pages |
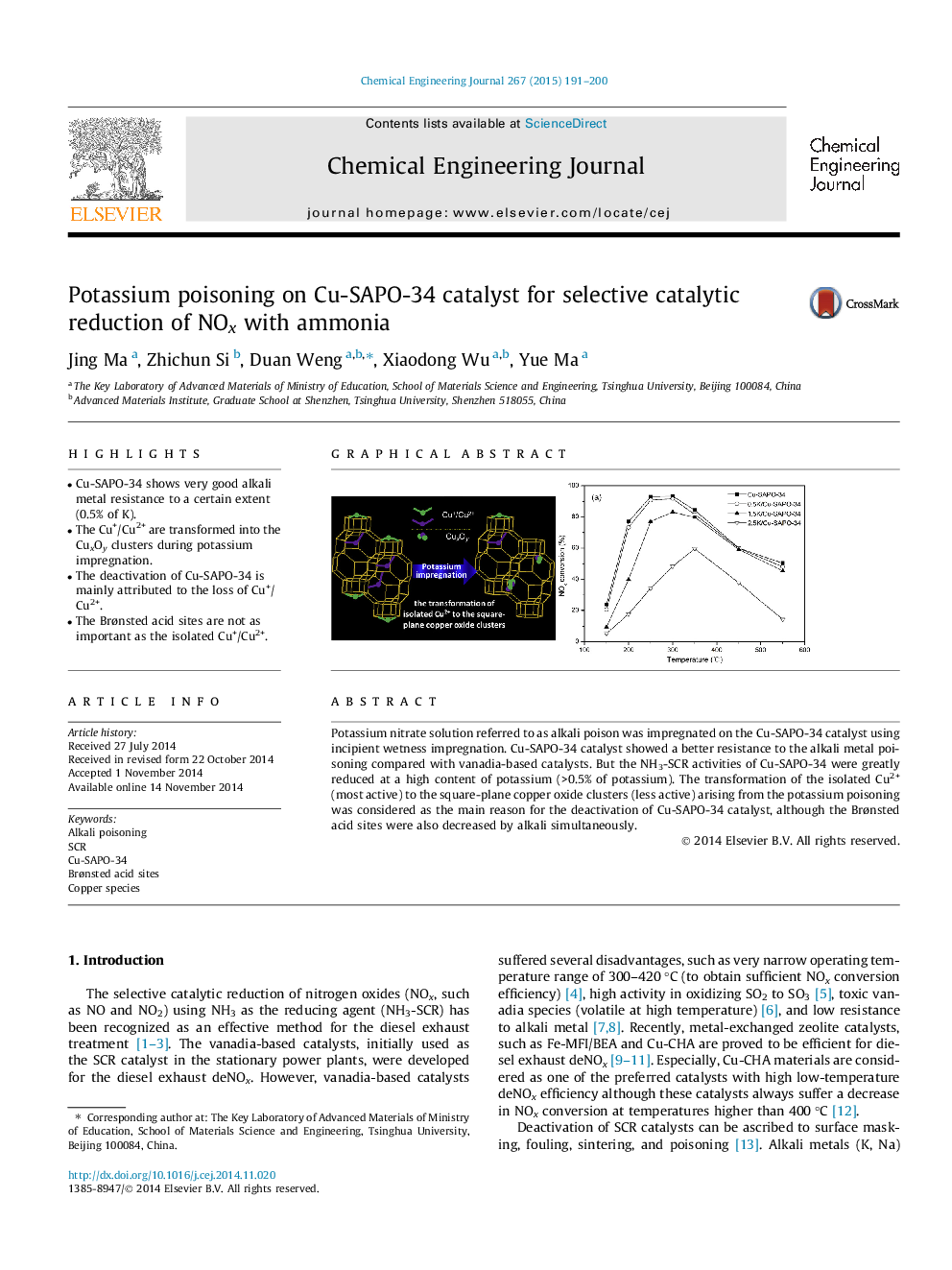
•Cu-SAPO-34 shows very good alkali metal resistance to a certain extent (0.5% of K).•The Cu+/Cu2+ are transformed into the CuxOy clusters during potassium impregnation.•The deactivation of Cu-SAPO-34 is mainly attributed to the loss of Cu+/Cu2+.•The Brønsted acid sites are not as important as the isolated Cu+/Cu2+.
Potassium nitrate solution referred to as alkali poison was impregnated on the Cu-SAPO-34 catalyst using incipient wetness impregnation. Cu-SAPO-34 catalyst showed a better resistance to the alkali metal poisoning compared with vanadia-based catalysts. But the NH3-SCR activities of Cu-SAPO-34 were greatly reduced at a high content of potassium (>0.5% of potassium). The transformation of the isolated Cu2+ (most active) to the square-plane copper oxide clusters (less active) arising from the potassium poisoning was considered as the main reason for the deactivation of Cu-SAPO-34 catalyst, although the Brønsted acid sites were also decreased by alkali simultaneously.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide