| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146628 | Chemical Engineering Journal | 2015 | 9 Pages |
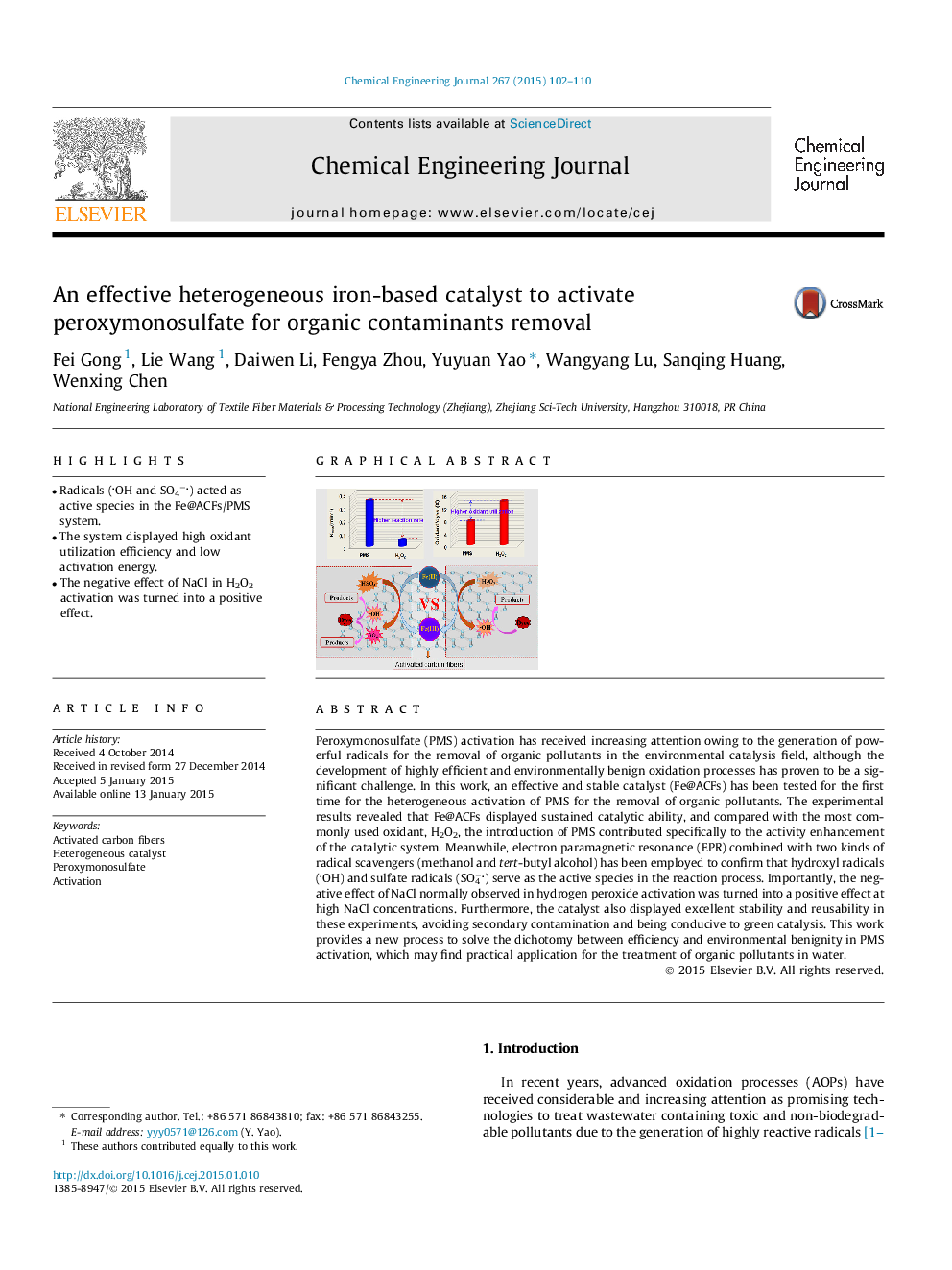
•Radicals (OH and SO4−) acted as active species in the Fe@ACFs/PMS system.•The system displayed high oxidant utilization efficiency and low activation energy.•The negative effect of NaCl in H2O2 activation was turned into a positive effect.
Peroxymonosulfate (PMS) activation has received increasing attention owing to the generation of powerful radicals for the removal of organic pollutants in the environmental catalysis field, although the development of highly efficient and environmentally benign oxidation processes has proven to be a significant challenge. In this work, an effective and stable catalyst (Fe@ACFs) has been tested for the first time for the heterogeneous activation of PMS for the removal of organic pollutants. The experimental results revealed that Fe@ACFs displayed sustained catalytic ability, and compared with the most commonly used oxidant, H2O2, the introduction of PMS contributed specifically to the activity enhancement of the catalytic system. Meanwhile, electron paramagnetic resonance (EPR) combined with two kinds of radical scavengers (methanol and tert-butyl alcohol) has been employed to confirm that hydroxyl radicals (OH) and sulfate radicals (SO4−) serve as the active species in the reaction process. Importantly, the negative effect of NaCl normally observed in hydrogen peroxide activation was turned into a positive effect at high NaCl concentrations. Furthermore, the catalyst also displayed excellent stability and reusability in these experiments, avoiding secondary contamination and being conducive to green catalysis. This work provides a new process to solve the dichotomy between efficiency and environmental benignity in PMS activation, which may find practical application for the treatment of organic pollutants in water.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide