| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146674 | Chemical Engineering Journal | 2015 | 9 Pages |
•Cu(II), Ni(II) and tannic acid were simultaneously removed by combined magnetic resins.•Complexing-bridging was ascribed as the predominant co-removal mechanism.•Hydrogen bond and ion exchange interaction were ascribed to TA adsorption onto MAEX.•Three adsorbates could be recovered with 0.1 M NaOH followed by 0.01 M HCl.
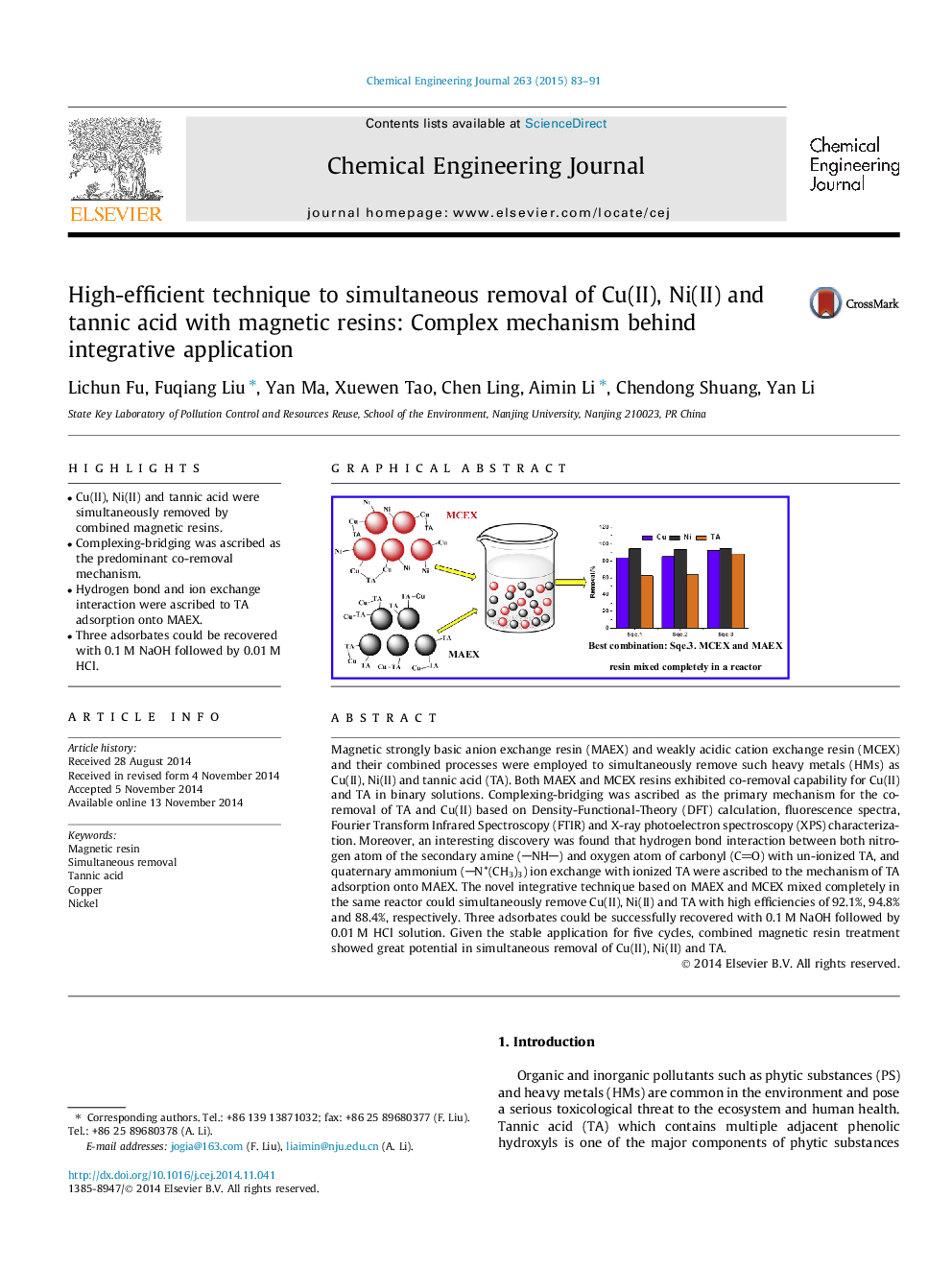
Magnetic strongly basic anion exchange resin (MAEX) and weakly acidic cation exchange resin (MCEX) and their combined processes were employed to simultaneously remove such heavy metals (HMs) as Cu(II), Ni(II) and tannic acid (TA). Both MAEX and MCEX resins exhibited co-removal capability for Cu(II) and TA in binary solutions. Complexing-bridging was ascribed as the primary mechanism for the co-removal of TA and Cu(II) based on Density-Functional-Theory (DFT) calculation, fluorescence spectra, Fourier Transform Infrared Spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS) characterization. Moreover, an interesting discovery was found that hydrogen bond interaction between both nitrogen atom of the secondary amine (NH) and oxygen atom of carbonyl (CO) with un-ionized TA, and quaternary ammonium (N+(CH3)3) ion exchange with ionized TA were ascribed to the mechanism of TA adsorption onto MAEX. The novel integrative technique based on MAEX and MCEX mixed completely in the same reactor could simultaneously remove Cu(II), Ni(II) and TA with high efficiencies of 92.1%, 94.8% and 88.4%, respectively. Three adsorbates could be successfully recovered with 0.1 M NaOH followed by 0.01 M HCl solution. Given the stable application for five cycles, combined magnetic resin treatment showed great potential in simultaneous removal of Cu(II), Ni(II) and TA.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide