| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146706 | Chemical Engineering Journal | 2015 | 7 Pages |
•Magnetically separable metalloporphyrin (M-FeTCP) was prepared via covalent conjugation.•M-FeTCP exhibited high capability in dye decoloration comparing to free FeTCP.•M-FeTCP could be easily recovered using a permanent magnetic bar.•M-FeTCP possesses stable recycle performance due to the covalent bond linkage.
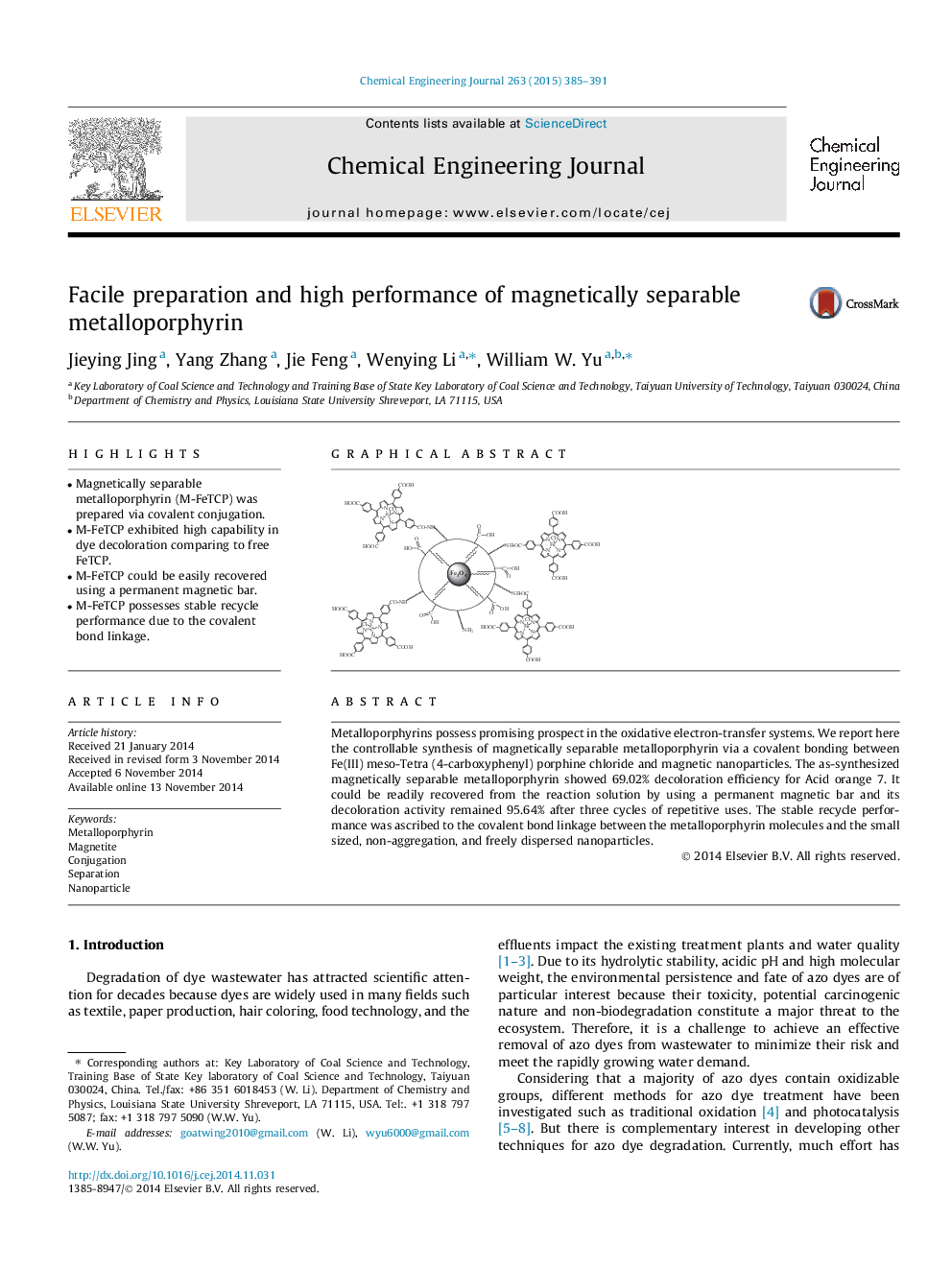
Metalloporphyrins possess promising prospect in the oxidative electron-transfer systems. We report here the controllable synthesis of magnetically separable metalloporphyrin via a covalent bonding between Fe(III) meso-Tetra (4-carboxyphenyl) porphine chloride and magnetic nanoparticles. The as-synthesized magnetically separable metalloporphyrin showed 69.02% decoloration efficiency for Acid orange 7. It could be readily recovered from the reaction solution by using a permanent magnetic bar and its decoloration activity remained 95.64% after three cycles of repetitive uses. The stable recycle performance was ascribed to the covalent bond linkage between the metalloporphyrin molecules and the small sized, non-aggregation, and freely dispersed nanoparticles.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide