| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146714 | Chemical Engineering Journal | 2015 | 8 Pages |
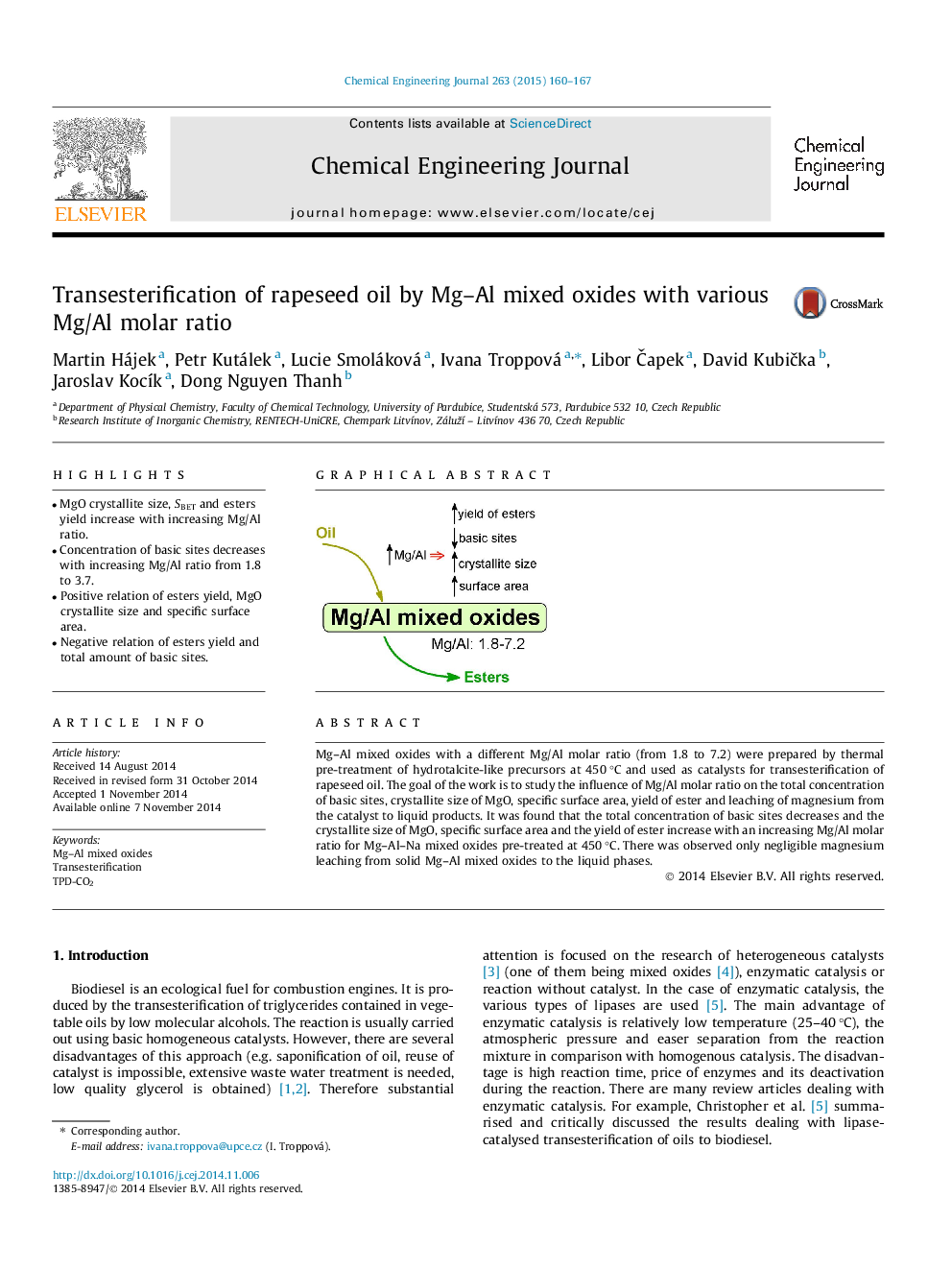
•MgO crystallite size, SBET and esters yield increase with increasing Mg/Al ratio.•Concentration of basic sites decreases with increasing Mg/Al ratio from 1.8 to 3.7.•Positive relation of esters yield, MgO crystallite size and specific surface area.•Negative relation of esters yield and total amount of basic sites.
Mg–Al mixed oxides with a different Mg/Al molar ratio (from 1.8 to 7.2) were prepared by thermal pre-treatment of hydrotalcite-like precursors at 450 °C and used as catalysts for transesterification of rapeseed oil. The goal of the work is to study the influence of Mg/Al molar ratio on the total concentration of basic sites, crystallite size of MgO, specific surface area, yield of ester and leaching of magnesium from the catalyst to liquid products. It was found that the total concentration of basic sites decreases and the crystallite size of MgO, specific surface area and the yield of ester increase with an increasing Mg/Al molar ratio for Mg–Al–Na mixed oxides pre-treated at 450 °C. There was observed only negligible magnesium leaching from solid Mg–Al mixed oxides to the liquid phases.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide