| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146736 | Chemical Engineering Journal | 2015 | 6 Pages |
•The solvent-free heterogeneous oxidation system is environmentally friendly.•PMo/ILMCM-41 prepared by grafting shows outstanding reusability in reaction.•Heterogeneous catalyst can easily separate by centrifugation after reaction.•PMo/ILMCM-41 possesses hydrophilic and hydrophobic properties.•PMo/ILMCM-41 can adsorb H2O2 and styrene simultaneously to improve the oxidation.
Styrene was selectively oxidized to 1,2-epoxyethylbenzene catalyzed by phosphomolybdic acid supported on ionic liquid modified MCM-41 using hydrogen peroxide as oxidant. The synthesized catalyst was characterized by XRD, FT-IR, and N2 adsorption–desorption analyses, and the results indicate that the sample retained mesoporous structure after ionic liquid-modified and immobilization of phosphomolybdic acid. Maximum activity was observed at a loading of 30 wt.% phosphomolybdic acid on ionic liquid modified MCM-41. Reaction conditions such as reaction time, temperature, amount of catalyst, and H2O2/styrene molar ratio were systematically optimized to obtain a maximum styrene conversion of 95.4%. 1,2-Epoxyethylbenzene selectivity of 90.2% when the reaction conditions were set to 3 h reaction time, 50 °C, 100 mg catalyst, and a H2O2/styrene molar ratio of 1.2. The heterogeneous catalyst is easily separated by centrifugation and was reused without deactivation after six runs.
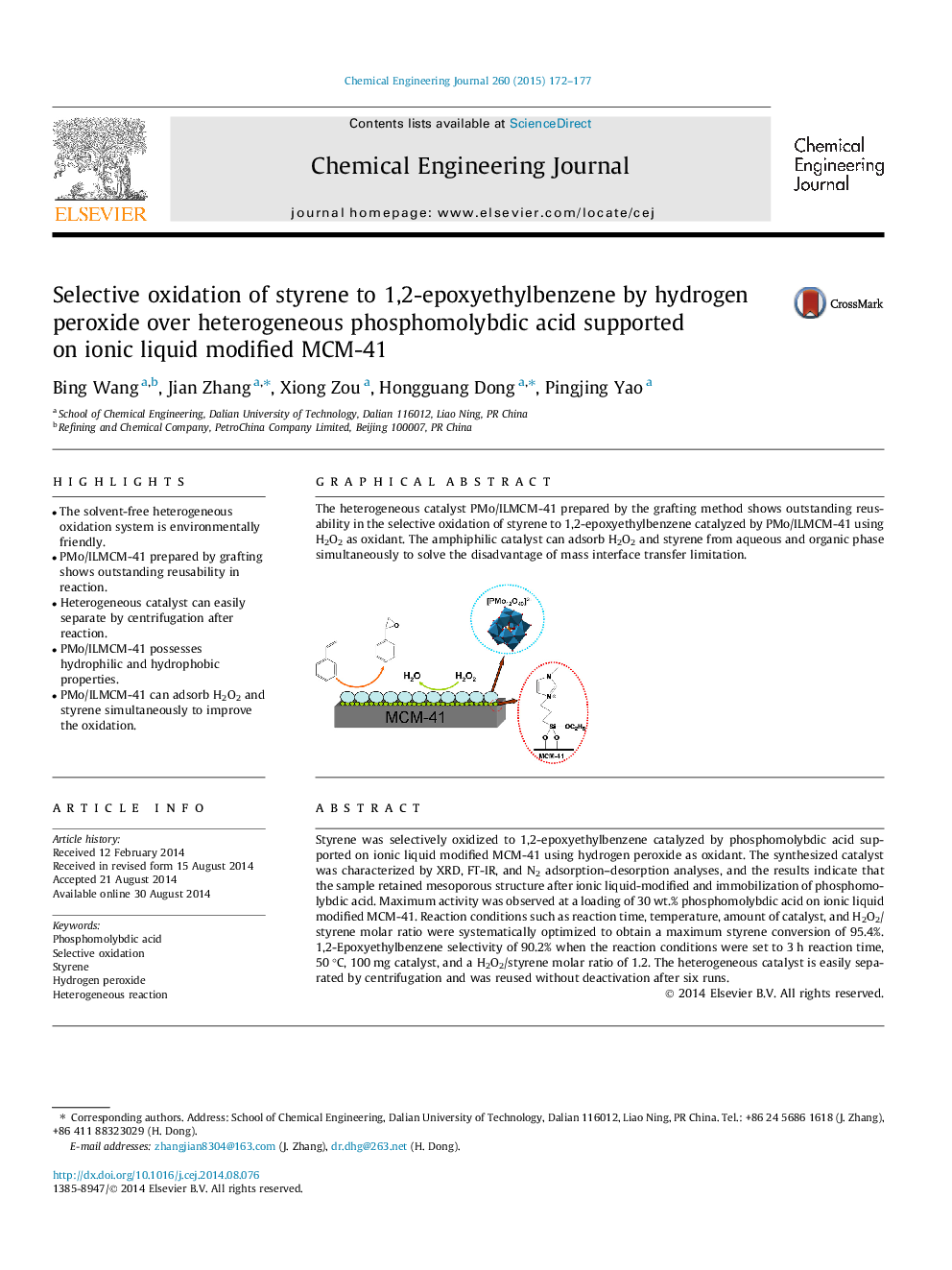
Graphical abstractThe heterogeneous catalyst PMo/ILMCM-41 prepared by the grafting method shows outstanding reusability in the selective oxidation of styrene to 1,2-epoxyethylbenzene catalyzed by PMo/ILMCM-41 using H2O2 as oxidant. The amphiphilic catalyst can adsorb H2O2 and styrene from aqueous and organic phase simultaneously to solve the disadvantage of mass interface transfer limitation.Figure optionsDownload full-size imageDownload as PowerPoint slide