| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146787 | Chemical Engineering Journal | 2015 | 9 Pages |
•70% of Zn2+ adsorption on BioSeNPs was completed in first minute of the reaction.•Adsorption of Zn2+ on BioSeNPs follows two-step mechanism at near-neutral pH.•Adsorption of Zn2+ on BioSeNPs follows ligand-like (type II) mechanism at low pH.•BioSeNPs loaded with Zn2+ have lower colloidal stability vis-a-vis BioSeNPs without Zn2+.
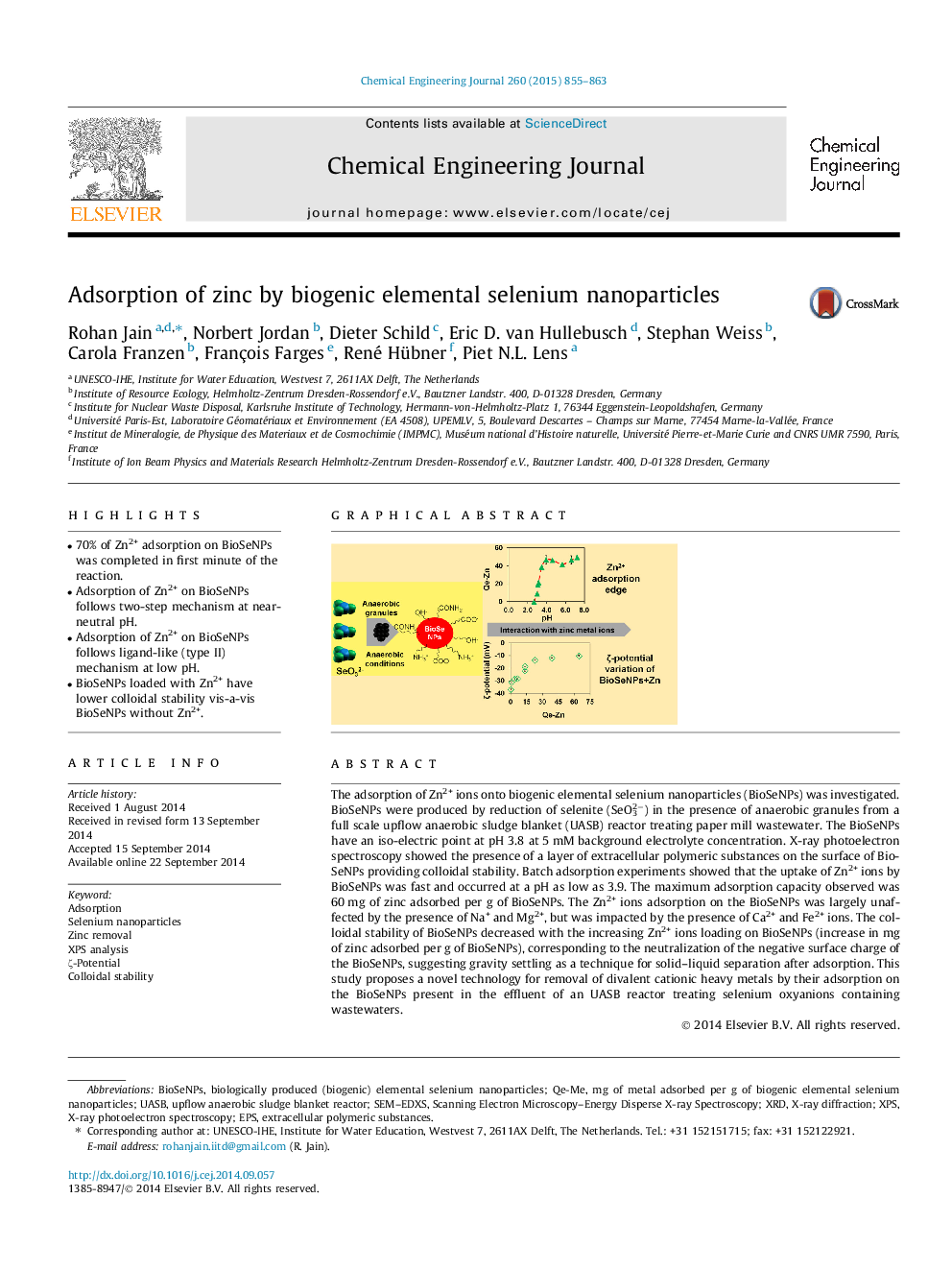
The adsorption of Zn2+ ions onto biogenic elemental selenium nanoparticles (BioSeNPs) was investigated. BioSeNPs were produced by reduction of selenite (SeO32−) in the presence of anaerobic granules from a full scale upflow anaerobic sludge blanket (UASB) reactor treating paper mill wastewater. The BioSeNPs have an iso-electric point at pH 3.8 at 5 mM background electrolyte concentration. X-ray photoelectron spectroscopy showed the presence of a layer of extracellular polymeric substances on the surface of BioSeNPs providing colloidal stability. Batch adsorption experiments showed that the uptake of Zn2+ ions by BioSeNPs was fast and occurred at a pH as low as 3.9. The maximum adsorption capacity observed was 60 mg of zinc adsorbed per g of BioSeNPs. The Zn2+ ions adsorption on the BioSeNPs was largely unaffected by the presence of Na+ and Mg2+, but was impacted by the presence of Ca2+ and Fe2+ ions. The colloidal stability of BioSeNPs decreased with the increasing Zn2+ ions loading on BioSeNPs (increase in mg of zinc adsorbed per g of BioSeNPs), corresponding to the neutralization of the negative surface charge of the BioSeNPs, suggesting gravity settling as a technique for solid–liquid separation after adsorption. This study proposes a novel technology for removal of divalent cationic heavy metals by their adsorption on the BioSeNPs present in the effluent of an UASB reactor treating selenium oxyanions containing wastewaters.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide