| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146797 | Chemical Engineering Journal | 2015 | 10 Pages |
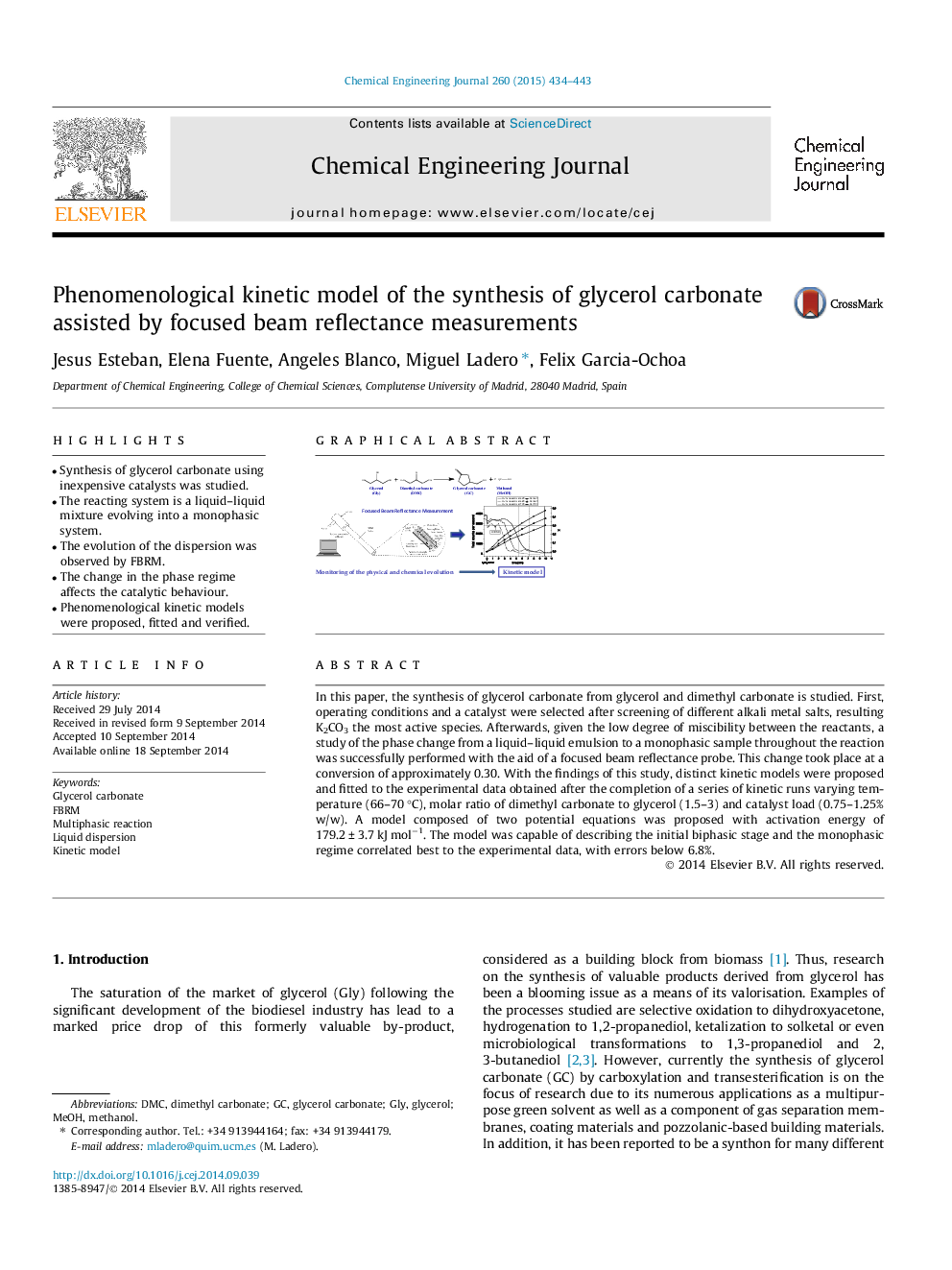
•Synthesis of glycerol carbonate using inexpensive catalysts was studied.•The reacting system is a liquid–liquid mixture evolving into a monophasic system.•The evolution of the dispersion was observed by FBRM.•The change in the phase regime affects the catalytic behaviour.•Phenomenological kinetic models were proposed, fitted and verified.
In this paper, the synthesis of glycerol carbonate from glycerol and dimethyl carbonate is studied. First, operating conditions and a catalyst were selected after screening of different alkali metal salts, resulting K2CO3 the most active species. Afterwards, given the low degree of miscibility between the reactants, a study of the phase change from a liquid–liquid emulsion to a monophasic sample throughout the reaction was successfully performed with the aid of a focused beam reflectance probe. This change took place at a conversion of approximately 0.30. With the findings of this study, distinct kinetic models were proposed and fitted to the experimental data obtained after the completion of a series of kinetic runs varying temperature (66–70 °C), molar ratio of dimethyl carbonate to glycerol (1.5–3) and catalyst load (0.75–1.25% w/w). A model composed of two potential equations was proposed with activation energy of 179.2 ± 3.7 kJ mol−1. The model was capable of describing the initial biphasic stage and the monophasic regime correlated best to the experimental data, with errors below 6.8%.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide