| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146989 | Chemical Engineering Journal | 2014 | 8 Pages |
•CO2 adsorption kinetic study on pore-expanded mesoporous silica (MCM-41) material.•Kinetic modeling under wide range of pressure and temperature conditions.•Detailed kinetic analysis is carried out to predict kinetic behavior of CO2 uptake.•Highlighted individual mechanistic steps that control the diffusion of CO2 uptake.
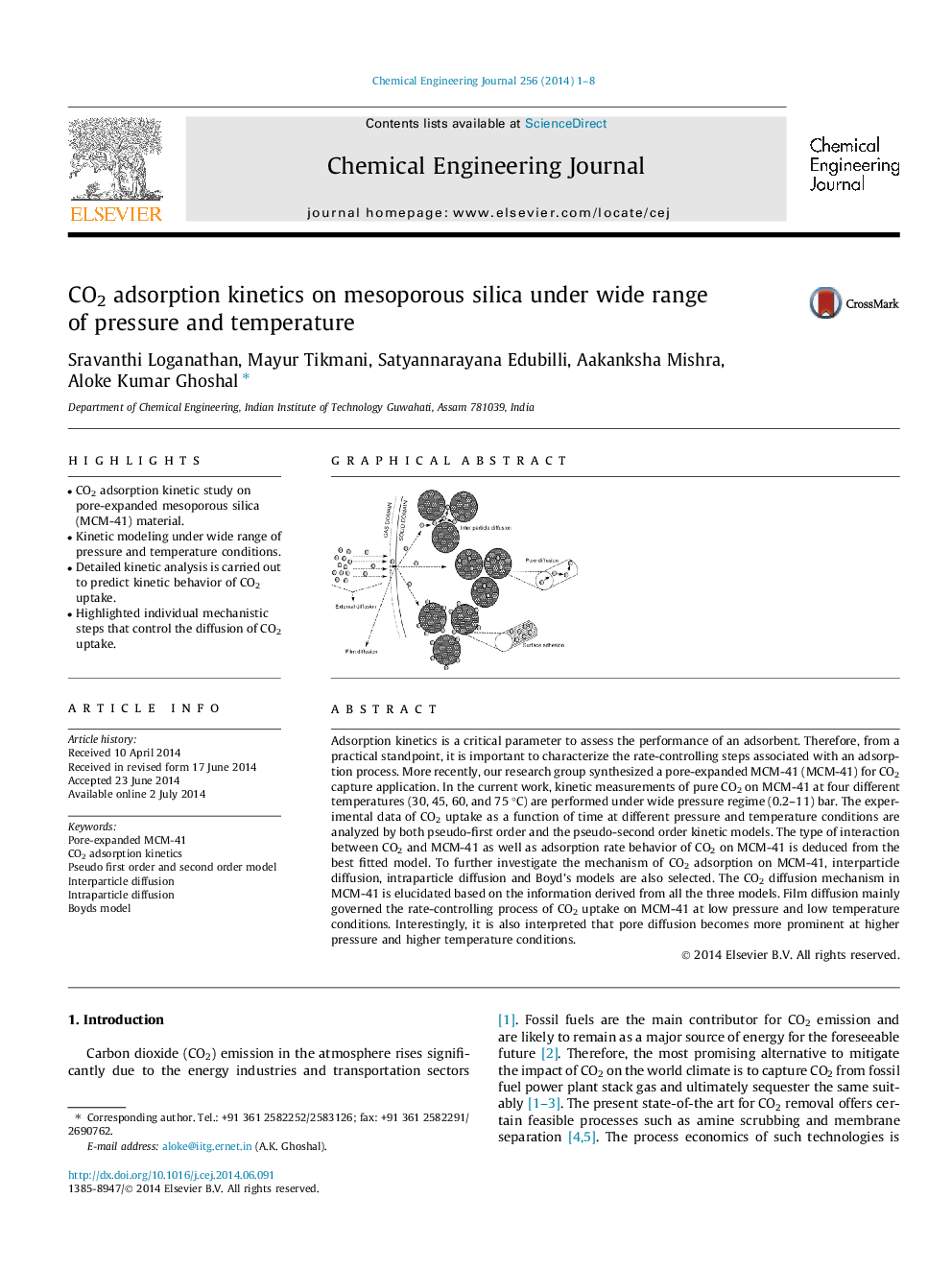
Adsorption kinetics is a critical parameter to assess the performance of an adsorbent. Therefore, from a practical standpoint, it is important to characterize the rate-controlling steps associated with an adsorption process. More recently, our research group synthesized a pore-expanded MCM-41 (MCM-41) for CO2 capture application. In the current work, kinetic measurements of pure CO2 on MCM-41 at four different temperatures (30, 45, 60, and 75 °C) are performed under wide pressure regime (0.2–11) bar. The experimental data of CO2 uptake as a function of time at different pressure and temperature conditions are analyzed by both pseudo-first order and the pseudo-second order kinetic models. The type of interaction between CO2 and MCM-41 as well as adsorption rate behavior of CO2 on MCM-41 is deduced from the best fitted model. To further investigate the mechanism of CO2 adsorption on MCM-41, interparticle diffusion, intraparticle diffusion and Boyd’s models are also selected. The CO2 diffusion mechanism in MCM-41 is elucidated based on the information derived from all the three models. Film diffusion mainly governed the rate-controlling process of CO2 uptake on MCM-41 at low pressure and low temperature conditions. Interestingly, it is also interpreted that pore diffusion becomes more prominent at higher pressure and higher temperature conditions.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide