| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147058 | Chemical Engineering Journal | 2014 | 7 Pages |
•Hybrid spheres Al/Fe impregnated with copper showed activity to reduction of nitrate in the liquid phase.•The iron-based catalysts were active in the reaction, with minor ammonia formation.•The preferential oxidation of iron maintains the copper activity for a longer period in the reaction.
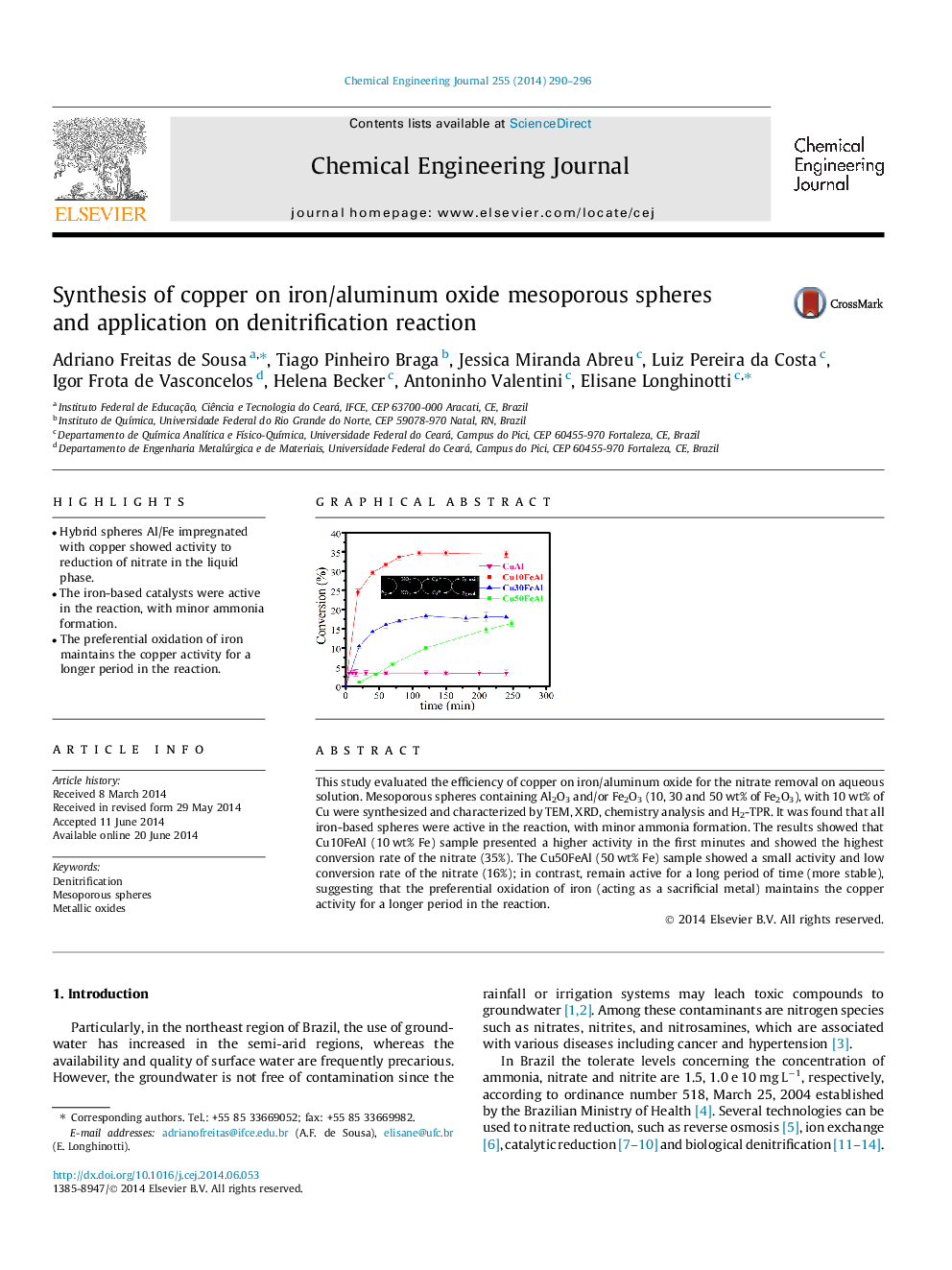
This study evaluated the efficiency of copper on iron/aluminum oxide for the nitrate removal on aqueous solution. Mesoporous spheres containing Al2O3 and/or Fe2O3 (10, 30 and 50 wt% of Fe2O3), with 10 wt% of Cu were synthesized and characterized by TEM, XRD, chemistry analysis and H2-TPR. It was found that all iron-based spheres were active in the reaction, with minor ammonia formation. The results showed that Cu10FeAl (10 wt% Fe) sample presented a higher activity in the first minutes and showed the highest conversion rate of the nitrate (35%). The Cu50FeAl (50 wt% Fe) sample showed a small activity and low conversion rate of the nitrate (16%); in contrast, remain active for a long period of time (more stable), suggesting that the preferential oxidation of iron (acting as a sacrificial metal) maintains the copper activity for a longer period in the reaction.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide