| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147151 | Chemical Engineering Journal | 2014 | 11 Pages |
•Ferric ions are the active centre responsible for the oxidation activity.•RH-10Fe was the best catalyst in oxidizing naphthalene into benign products.•Textural properties and surface morphology influenced the catalytic activity.•Free-radical mechanism was occurring for this catalyst system.•Occurrence of loosely bounded ferric ions was minimal under the studied condition.
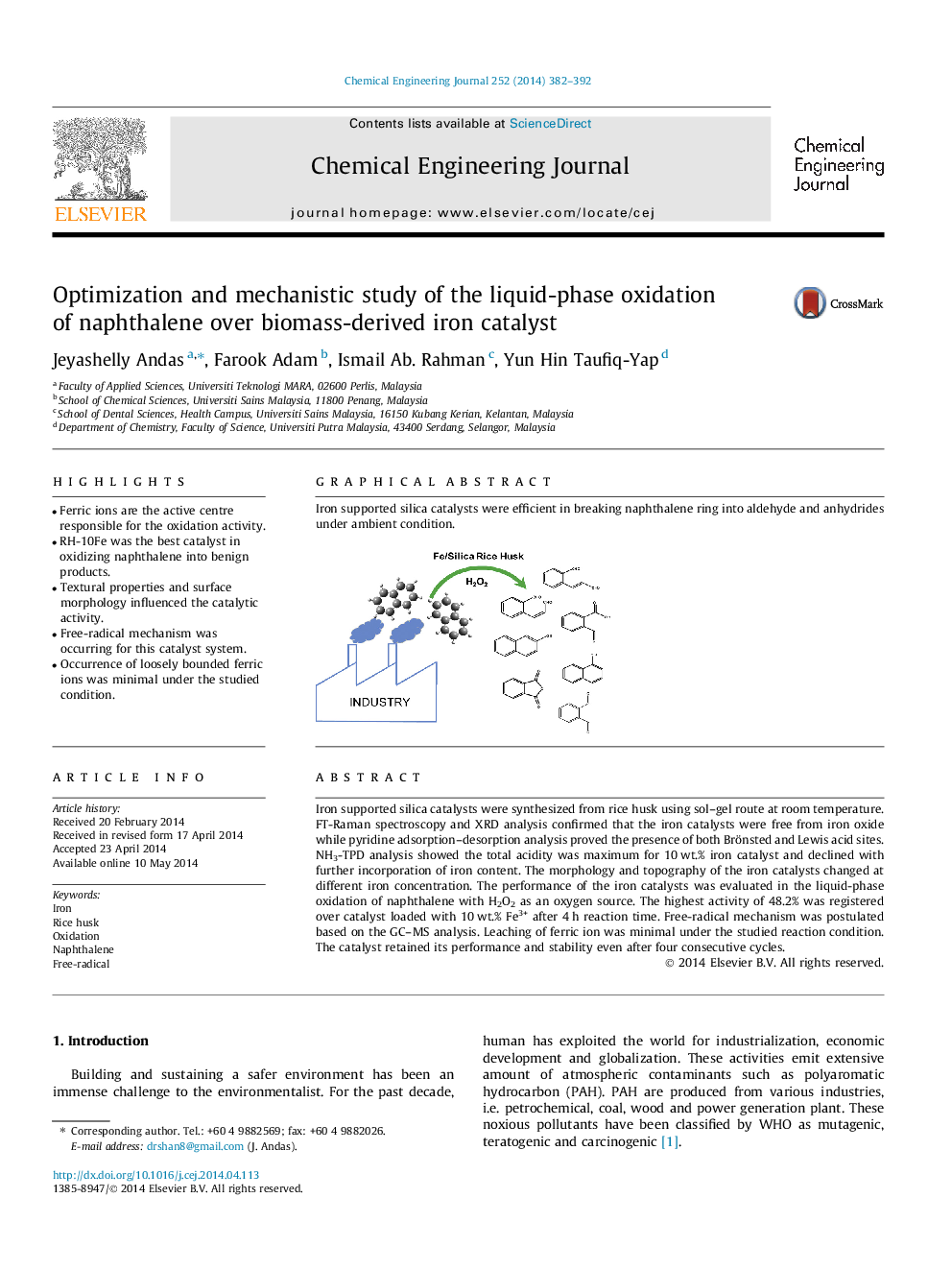
Iron supported silica catalysts were synthesized from rice husk using sol–gel route at room temperature. FT-Raman spectroscopy and XRD analysis confirmed that the iron catalysts were free from iron oxide while pyridine adsorption–desorption analysis proved the presence of both Brönsted and Lewis acid sites. NH3-TPD analysis showed the total acidity was maximum for 10 wt.% iron catalyst and declined with further incorporation of iron content. The morphology and topography of the iron catalysts changed at different iron concentration. The performance of the iron catalysts was evaluated in the liquid-phase oxidation of naphthalene with H2O2 as an oxygen source. The highest activity of 48.2% was registered over catalyst loaded with 10 wt.% Fe3+ after 4 h reaction time. Free-radical mechanism was postulated based on the GC–MS analysis. Leaching of ferric ion was minimal under the studied reaction condition. The catalyst retained its performance and stability even after four consecutive cycles.
Graphical abstractIron supported silica catalysts were efficient in breaking naphthalene ring into aldehyde and anhydrides under ambient condition.Figure optionsDownload full-size imageDownload as PowerPoint slide