| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147241 | Chemical Engineering Journal | 2014 | 10 Pages |
•Proposed mechanism of interaction between hydrotalcite and surfactants.•Organocompounds, HT-iron oxide interspersed with surfactants, are novel in literature.•Application of HT-iron oxide interspersed with surfactants on adsorption of cationic dye.•Study of the zeta potential versus pH indicates that the hydrophobic interaction acts on the adsorption process.
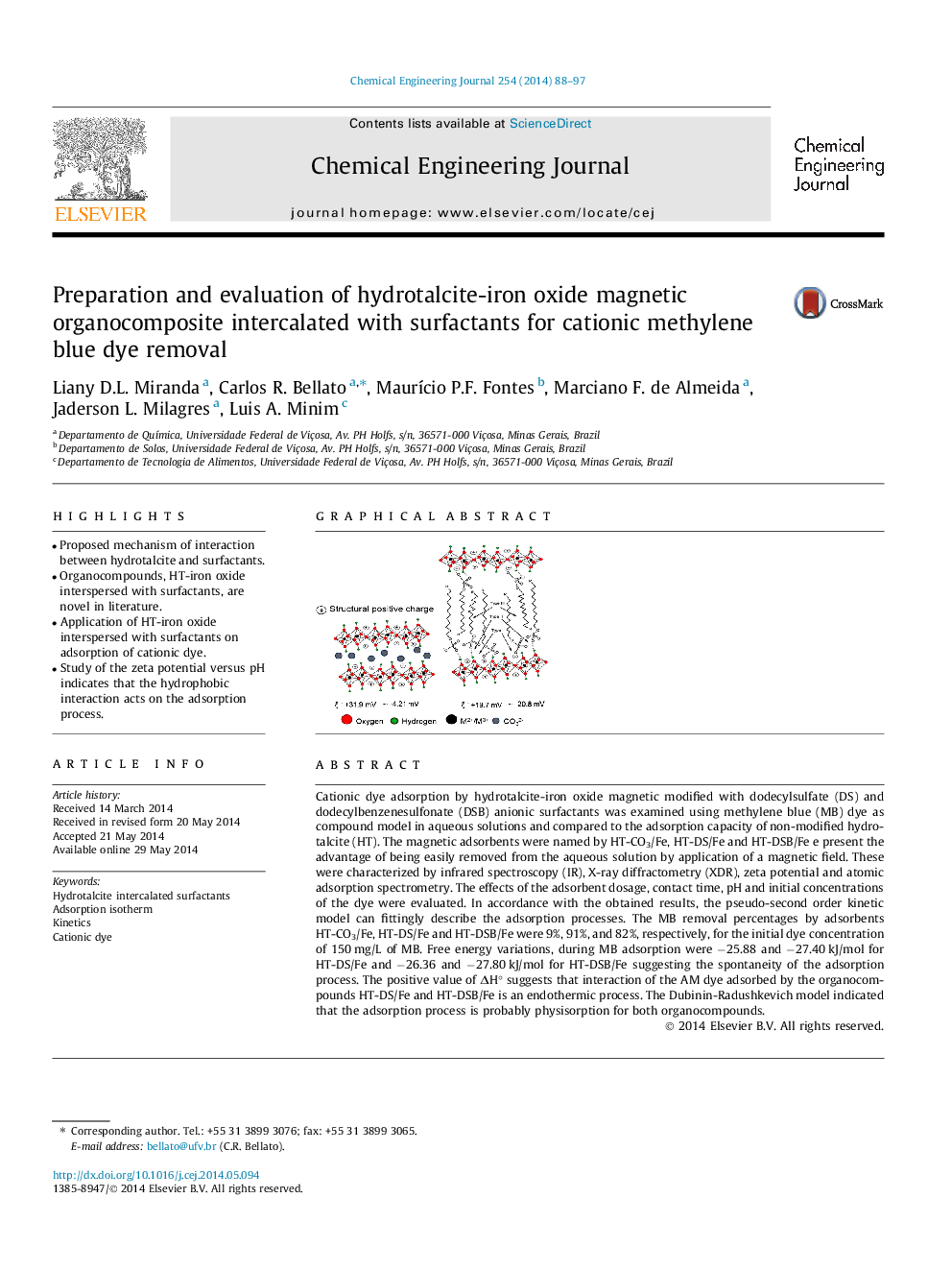
Cationic dye adsorption by hydrotalcite-iron oxide magnetic modified with dodecylsulfate (DS) and dodecylbenzenesulfonate (DSB) anionic surfactants was examined using methylene blue (MB) dye as compound model in aqueous solutions and compared to the adsorption capacity of non-modified hydrotalcite (HT). The magnetic adsorbents were named by HT-CO3/Fe, HT-DS/Fe and HT-DSB/Fe e present the advantage of being easily removed from the aqueous solution by application of a magnetic field. These were characterized by infrared spectroscopy (IR), X-ray diffractometry (XDR), zeta potential and atomic adsorption spectrometry. The effects of the adsorbent dosage, contact time, pH and initial concentrations of the dye were evaluated. In accordance with the obtained results, the pseudo-second order kinetic model can fittingly describe the adsorption processes. The MB removal percentages by adsorbents HT-CO3/Fe, HT-DS/Fe and HT-DSB/Fe were 9%, 91%, and 82%, respectively, for the initial dye concentration of 150 mg/L of MB. Free energy variations, during MB adsorption were −25.88 and −27.40 kJ/mol for HT-DS/Fe and −26.36 and −27.80 kJ/mol for HT-DSB/Fe suggesting the spontaneity of the adsorption process. The positive value of ΔH° suggests that interaction of the AM dye adsorbed by the organocompounds HT-DS/Fe and HT-DSB/Fe is an endothermic process. The Dubinin-Radushkevich model indicated that the adsorption process is probably physisorption for both organocompounds.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide