| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147252 | Chemical Engineering Journal | 2014 | 10 Pages |
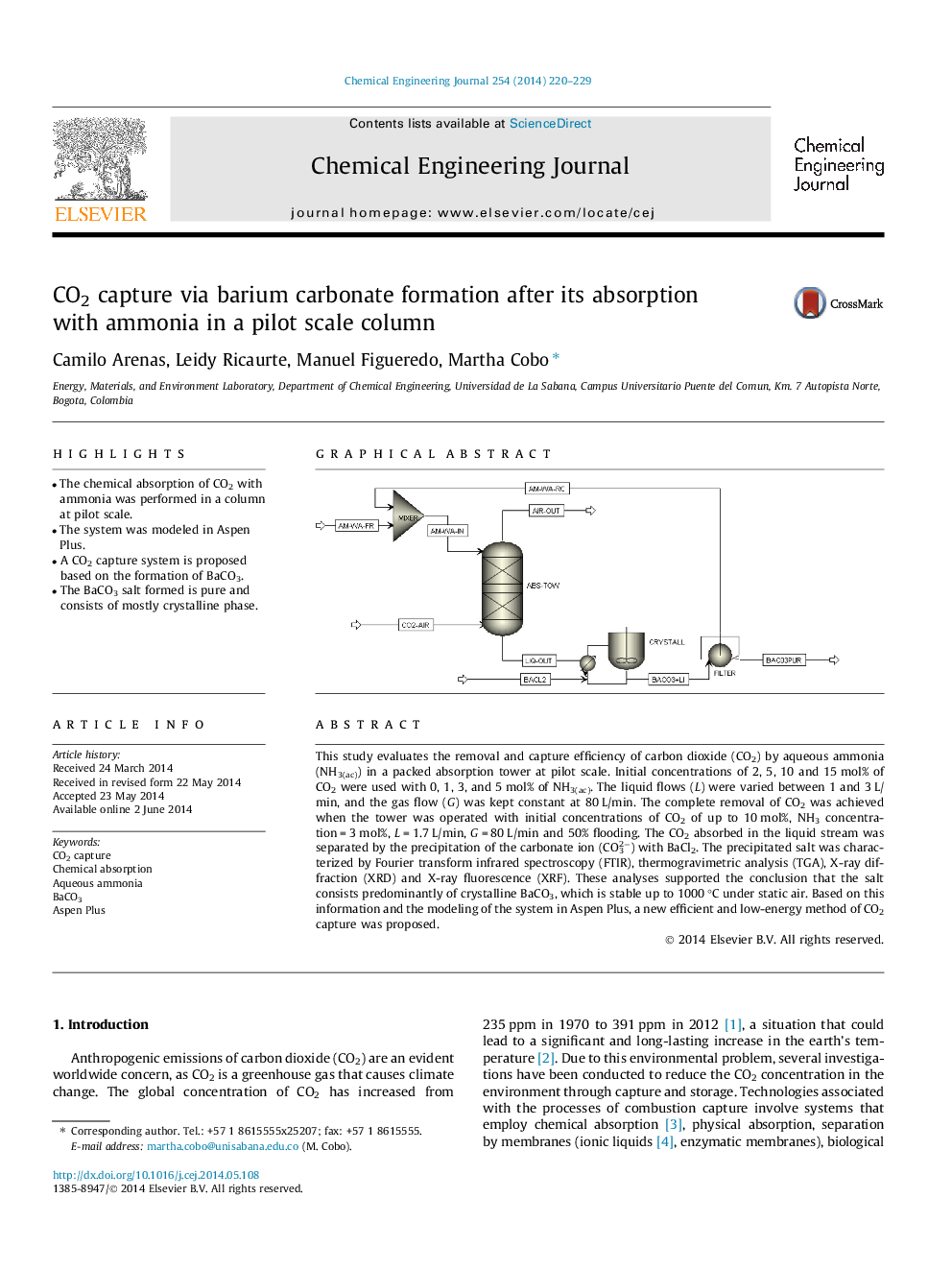
•The chemical absorption of CO2 with ammonia was performed in a column at pilot scale.•The system was modeled in Aspen Plus.•A CO2 capture system is proposed based on the formation of BaCO3.•The BaCO3 salt formed is pure and consists of mostly crystalline phase.
This study evaluates the removal and capture efficiency of carbon dioxide (CO2) by aqueous ammonia (NH3(ac)) in a packed absorption tower at pilot scale. Initial concentrations of 2, 5, 10 and 15 mol% of CO2 were used with 0, 1, 3, and 5 mol% of NH3(ac). The liquid flows (L) were varied between 1 and 3 L/min, and the gas flow (G) was kept constant at 80 L/min. The complete removal of CO2 was achieved when the tower was operated with initial concentrations of CO2 of up to 10 mol%, NH3 concentration = 3 mol%, L = 1.7 L/min, G = 80 L/min and 50% flooding. The CO2 absorbed in the liquid stream was separated by the precipitation of the carbonate ion (CO32−) with BaCl2. The precipitated salt was characterized by Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), X-ray diffraction (XRD) and X-ray fluorescence (XRF). These analyses supported the conclusion that the salt consists predominantly of crystalline BaCO3, which is stable up to 1000 °C under static air. Based on this information and the modeling of the system in Aspen Plus, a new efficient and low-energy method of CO2 capture was proposed.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide