| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147257 | Chemical Engineering Journal | 2014 | 7 Pages |
•A biomimetic catalyst was synthesized by intercalating hemin into clay mineral.•The amount of hemin on montmorillonite could reach as high as over 9% by weight.•Superior reactivity could be retained by hemin–montmorillonite bioconjugate.
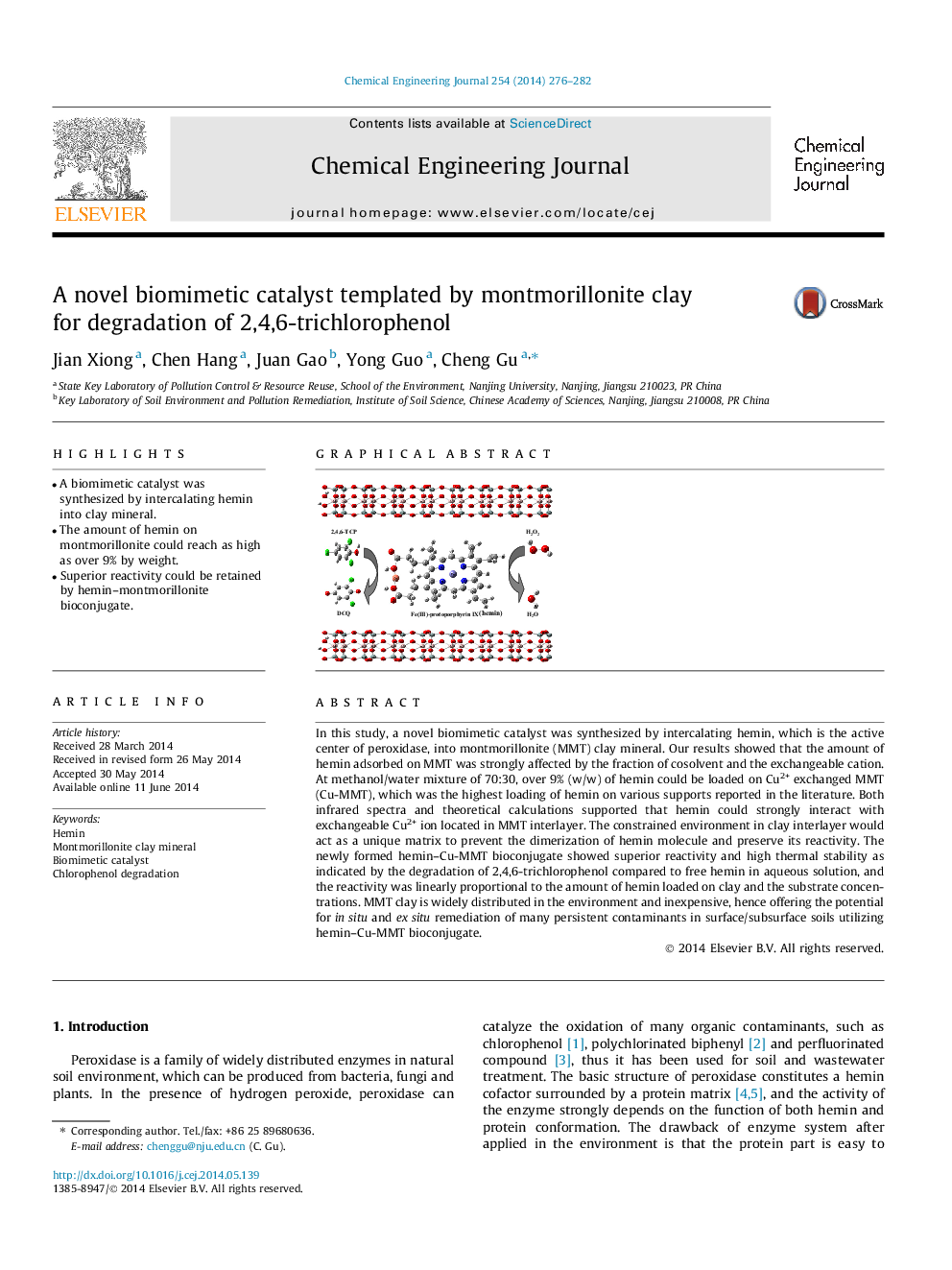
In this study, a novel biomimetic catalyst was synthesized by intercalating hemin, which is the active center of peroxidase, into montmorillonite (MMT) clay mineral. Our results showed that the amount of hemin adsorbed on MMT was strongly affected by the fraction of cosolvent and the exchangeable cation. At methanol/water mixture of 70:30, over 9% (w/w) of hemin could be loaded on Cu2+ exchanged MMT (Cu-MMT), which was the highest loading of hemin on various supports reported in the literature. Both infrared spectra and theoretical calculations supported that hemin could strongly interact with exchangeable Cu2+ ion located in MMT interlayer. The constrained environment in clay interlayer would act as a unique matrix to prevent the dimerization of hemin molecule and preserve its reactivity. The newly formed hemin–Cu-MMT bioconjugate showed superior reactivity and high thermal stability as indicated by the degradation of 2,4,6-trichlorophenol compared to free hemin in aqueous solution, and the reactivity was linearly proportional to the amount of hemin loaded on clay and the substrate concentrations. MMT clay is widely distributed in the environment and inexpensive, hence offering the potential for in situ and ex situ remediation of many persistent contaminants in surface/subsurface soils utilizing hemin–Cu-MMT bioconjugate.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide