| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147259 | Chemical Engineering Journal | 2014 | 9 Pages |
•We developed a novel CF–UF process for antimony (III) removal.•The antimony (III) removal efficiency was evaluated to optimize the process parameters.•The membrane rejection percentage for antimony (III) removal was higher than 90%.•The fate of antimony (III) in the CF–UF process was quantitatively investigated.
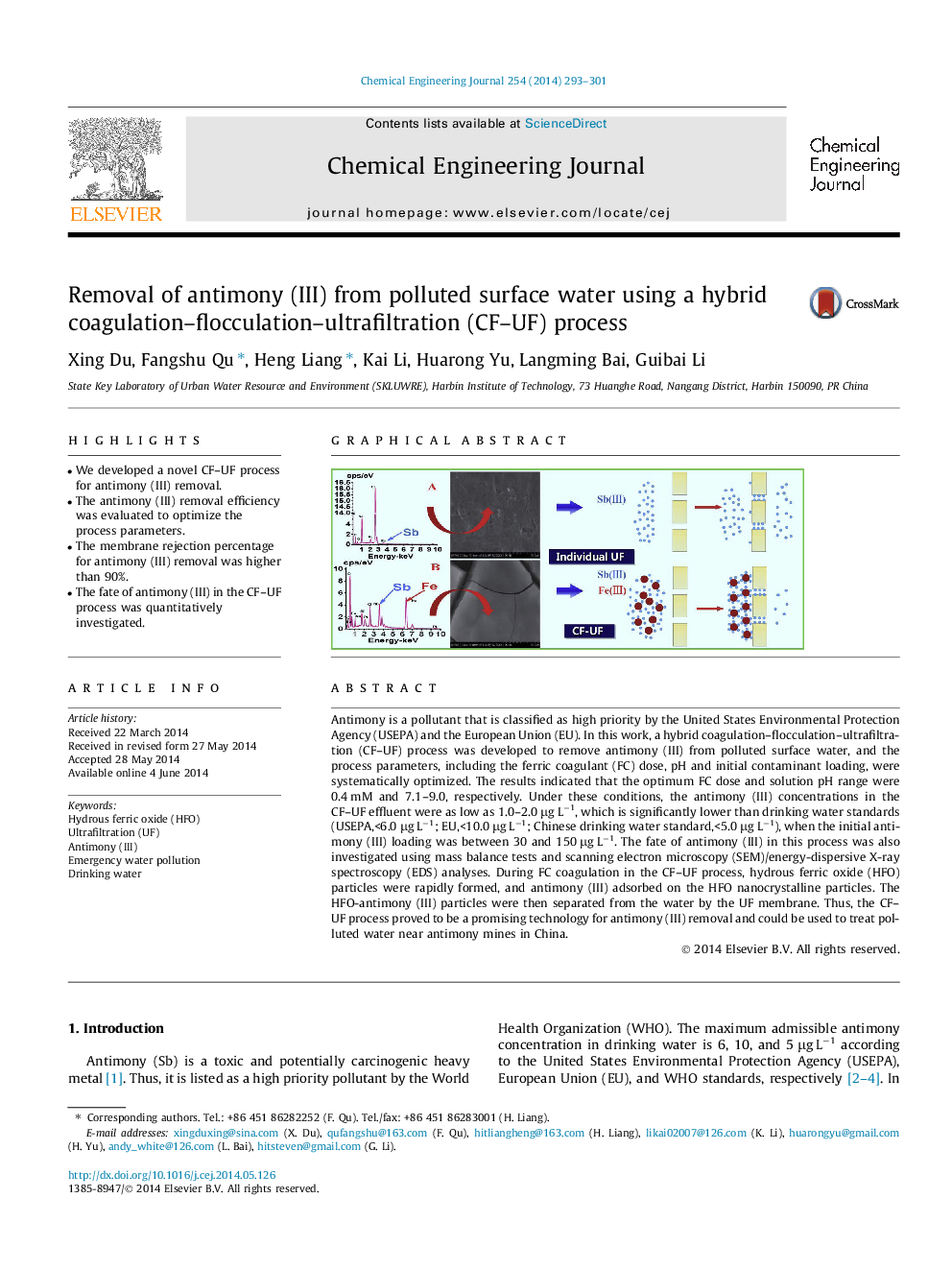
Antimony is a pollutant that is classified as high priority by the United States Environmental Protection Agency (USEPA) and the European Union (EU). In this work, a hybrid coagulation–flocculation–ultrafiltration (CF–UF) process was developed to remove antimony (III) from polluted surface water, and the process parameters, including the ferric coagulant (FC) dose, pH and initial contaminant loading, were systematically optimized. The results indicated that the optimum FC dose and solution pH range were 0.4 mM and 7.1–9.0, respectively. Under these conditions, the antimony (III) concentrations in the CF–UF effluent were as low as 1.0–2.0 μg L−1, which is significantly lower than drinking water standards (USEPA,<6.0 μg L−1; EU,<10.0 μg L−1; Chinese drinking water standard,<5.0 μg L−1), when the initial antimony (III) loading was between 30 and 150 μg L−1. The fate of antimony (III) in this process was also investigated using mass balance tests and scanning electron microscopy (SEM)/energy-dispersive X-ray spectroscopy (EDS) analyses. During FC coagulation in the CF–UF process, hydrous ferric oxide (HFO) particles were rapidly formed, and antimony (III) adsorbed on the HFO nanocrystalline particles. The HFO-antimony (III) particles were then separated from the water by the UF membrane. Thus, the CF–UF process proved to be a promising technology for antimony (III) removal and could be used to treat polluted water near antimony mines in China.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide