| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147330 | Chemical Engineering Journal | 2014 | 7 Pages |
•I-THMs formation from iopamidol favored AOM rather than HA/FA during monochloramination.•Br− exhibited higher activity to form I-THMs from I− pathway than iopamidol pathway.•I-THMs formed rapidly from iodide but relatively slow from iopamidol.•I-THMs yields positive correlated with SUVA in I− pathway while iopamidol negative.
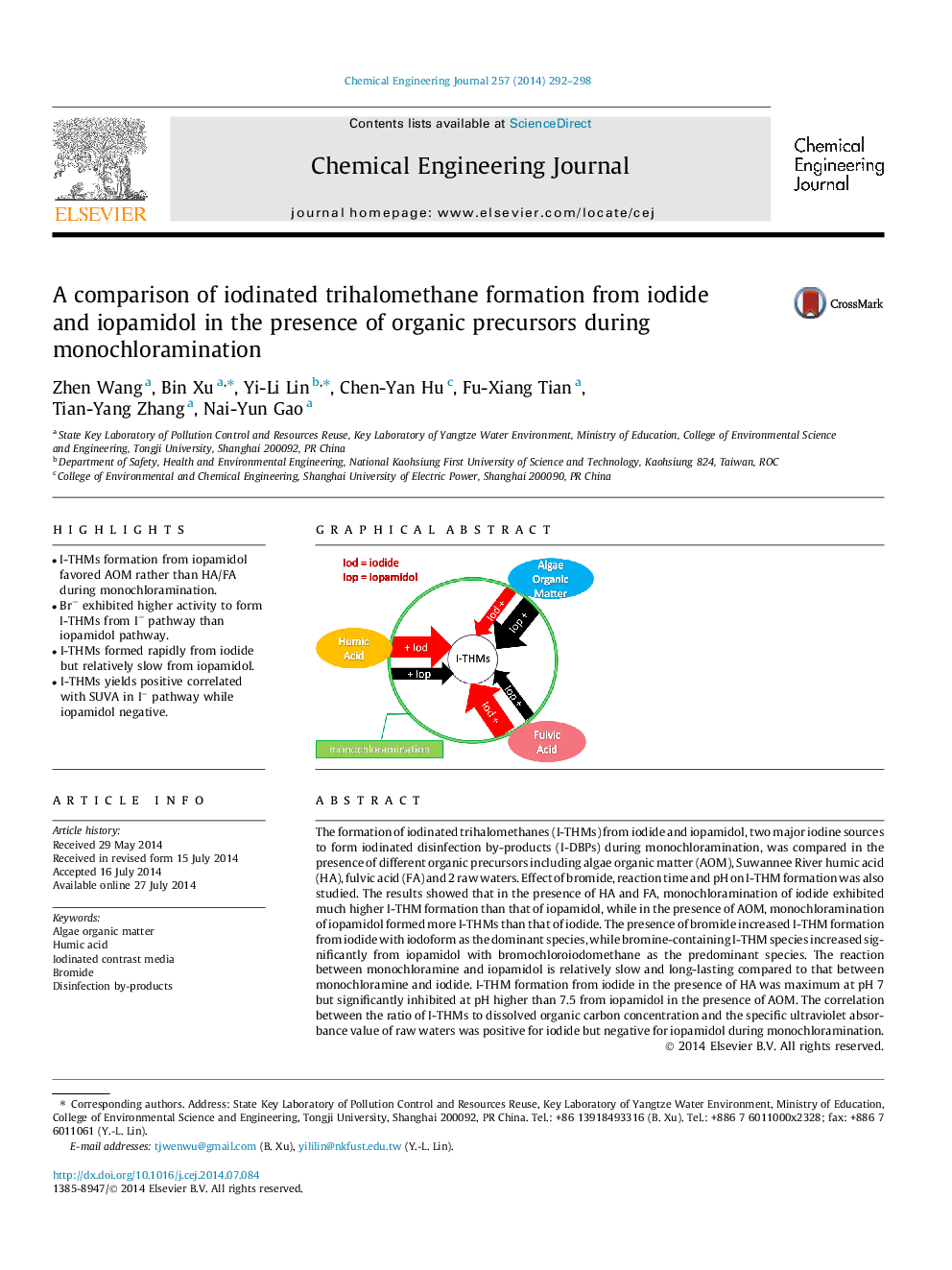
The formation of iodinated trihalomethanes (I-THMs) from iodide and iopamidol, two major iodine sources to form iodinated disinfection by-products (I-DBPs) during monochloramination, was compared in the presence of different organic precursors including algae organic matter (AOM), Suwannee River humic acid (HA), fulvic acid (FA) and 2 raw waters. Effect of bromide, reaction time and pH on I-THM formation was also studied. The results showed that in the presence of HA and FA, monochloramination of iodide exhibited much higher I-THM formation than that of iopamidol, while in the presence of AOM, monochloramination of iopamidol formed more I-THMs than that of iodide. The presence of bromide increased I-THM formation from iodide with iodoform as the dominant species, while bromine-containing I-THM species increased significantly from iopamidol with bromochloroiodomethane as the predominant species. The reaction between monochloramine and iopamidol is relatively slow and long-lasting compared to that between monochloramine and iodide. I-THM formation from iodide in the presence of HA was maximum at pH 7 but significantly inhibited at pH higher than 7.5 from iopamidol in the presence of AOM. The correlation between the ratio of I-THMs to dissolved organic carbon concentration and the specific ultraviolet absorbance value of raw waters was positive for iodide but negative for iopamidol during monochloramination.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide