| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147339 | Chemical Engineering Journal | 2014 | 8 Pages |
•A metalloporphyrinic metal–organic framework catalyst was very active for selective oxidation.•The crystal structure and catalysis-promoted environment facilitates the selective oxidation.•An insight into the catalytic mechanism of HCO3− was gained according to a theoretical study.
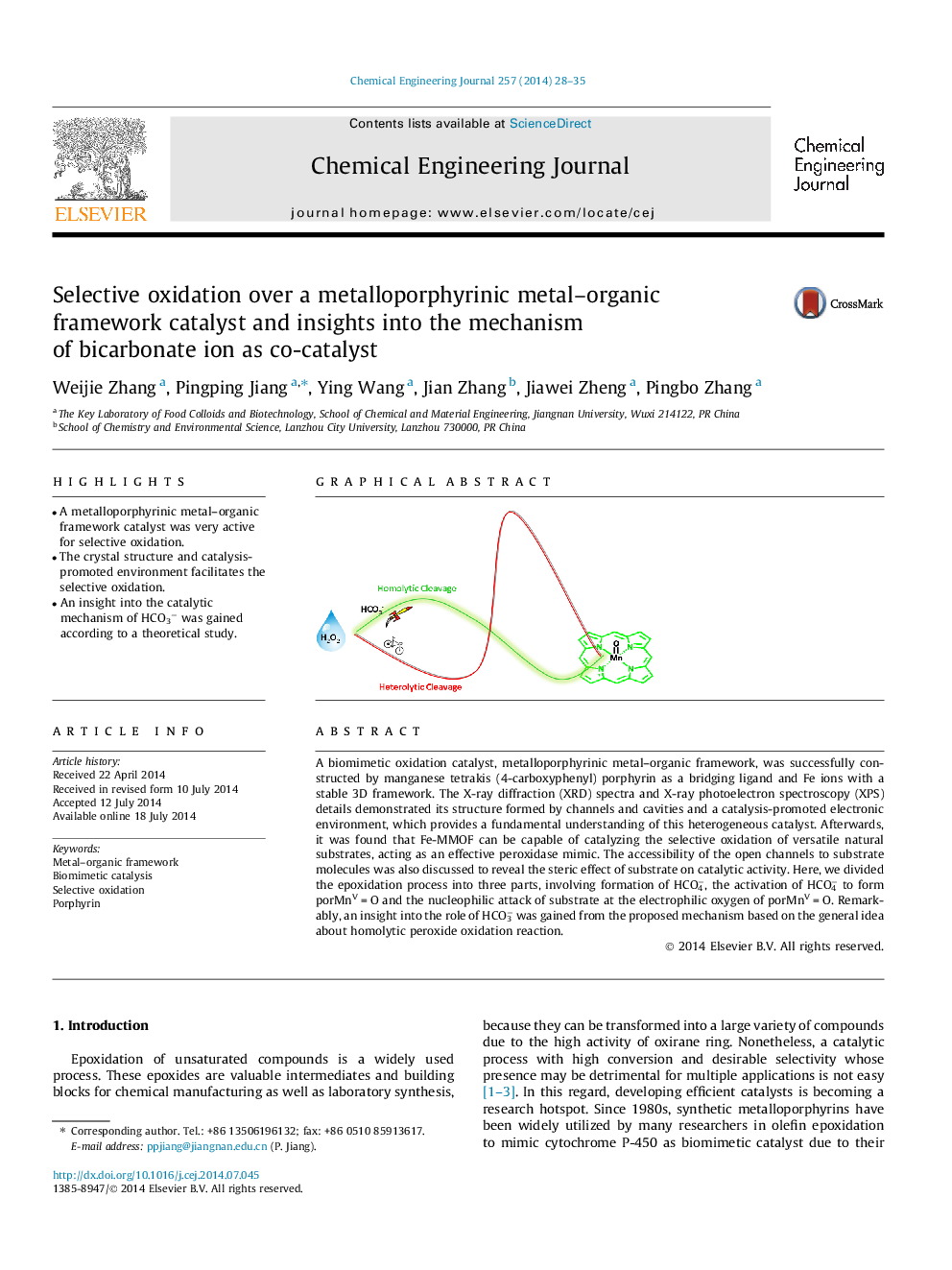
A biomimetic oxidation catalyst, metalloporphyrinic metal–organic framework, was successfully constructed by manganese tetrakis (4-carboxyphenyl) porphyrin as a bridging ligand and Fe ions with a stable 3D framework. The X-ray diffraction (XRD) spectra and X-ray photoelectron spectroscopy (XPS) details demonstrated its structure formed by channels and cavities and a catalysis-promoted electronic environment, which provides a fundamental understanding of this heterogeneous catalyst. Afterwards, it was found that Fe-MMOF can be capable of catalyzing the selective oxidation of versatile natural substrates, acting as an effective peroxidase mimic. The accessibility of the open channels to substrate molecules was also discussed to reveal the steric effect of substrate on catalytic activity. Here, we divided the epoxidation process into three parts, involving formation of HCO4−, the activation of HCO4− to form porMnV = O and the nucleophilic attack of substrate at the electrophilic oxygen of porMnV = O. Remarkably, an insight into the role of HCO3− was gained from the proposed mechanism based on the general idea about homolytic peroxide oxidation reaction.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide