| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147395 | Chemical Engineering Journal | 2014 | 5 Pages |
•Regular samplings allows following the degradation of a pollutant in photocatalysis.•The volume variation due to sampling influences the kinetic rate constant.•The error on the reaction rate constant depends on the relative volume variation.•Researchers performing a kinetic study on photocatalytic film have to be careful.
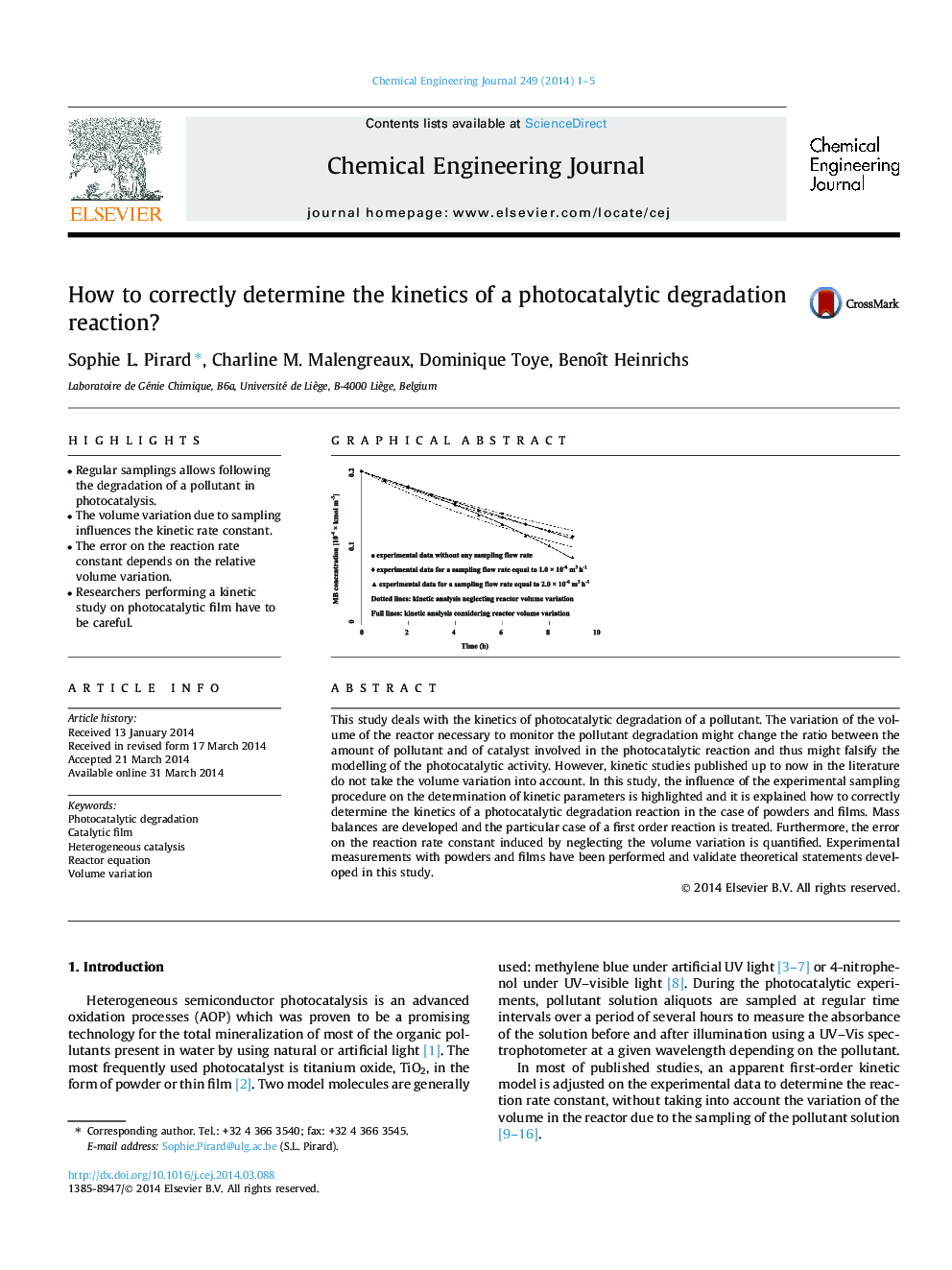
This study deals with the kinetics of photocatalytic degradation of a pollutant. The variation of the volume of the reactor necessary to monitor the pollutant degradation might change the ratio between the amount of pollutant and of catalyst involved in the photocatalytic reaction and thus might falsify the modelling of the photocatalytic activity. However, kinetic studies published up to now in the literature do not take the volume variation into account. In this study, the influence of the experimental sampling procedure on the determination of kinetic parameters is highlighted and it is explained how to correctly determine the kinetics of a photocatalytic degradation reaction in the case of powders and films. Mass balances are developed and the particular case of a first order reaction is treated. Furthermore, the error on the reaction rate constant induced by neglecting the volume variation is quantified. Experimental measurements with powders and films have been performed and validate theoretical statements developed in this study.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide