| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147434 | Chemical Engineering Journal | 2014 | 7 Pages |
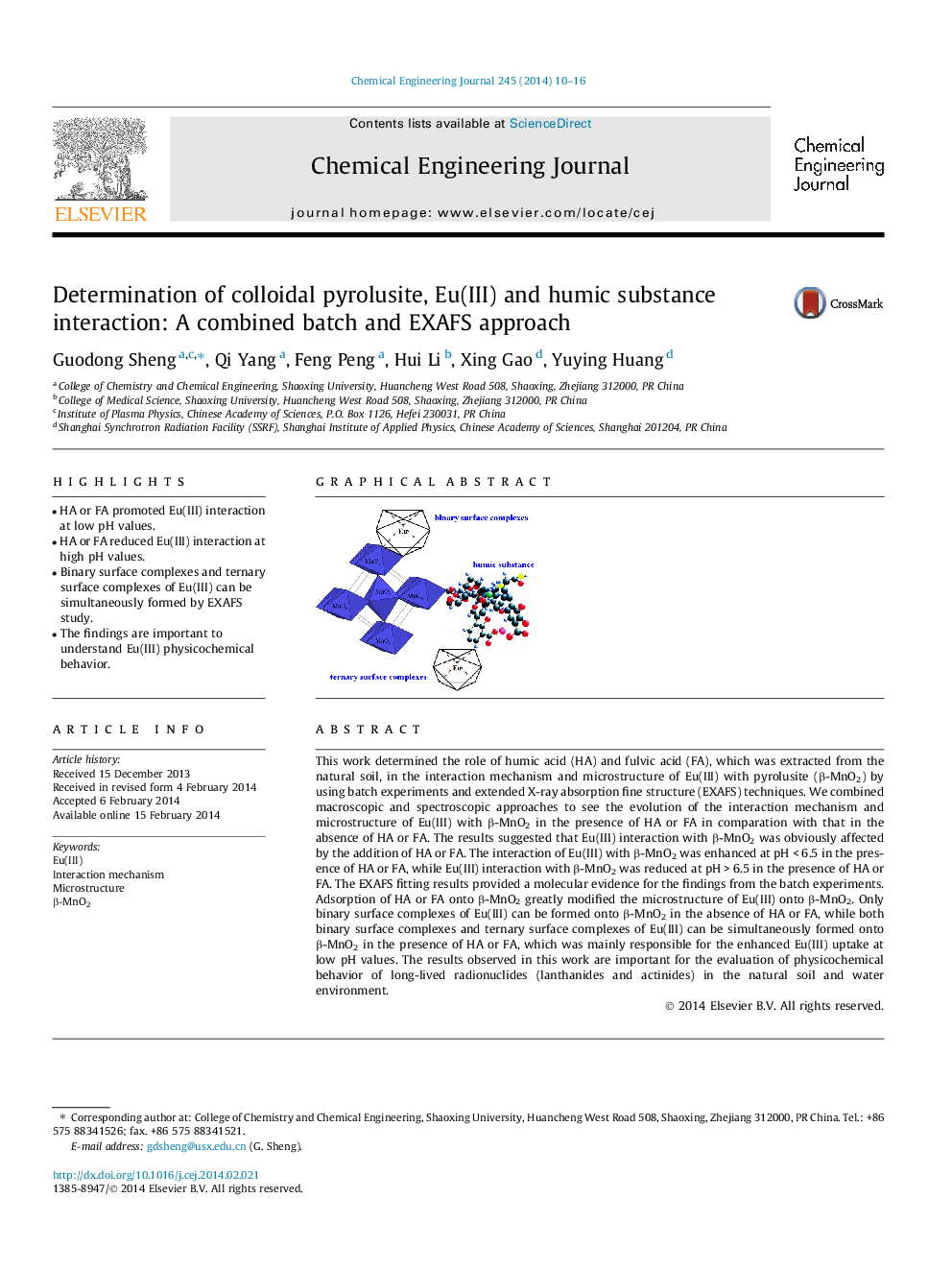
•HA or FA promoted Eu(III) interaction at low pH values.•HA or FA reduced Eu(III) interaction at high pH values.•Binary surface complexes and ternary surface complexes of Eu(III) can be simultaneously formed by EXAFS study.•The findings are important to understand Eu(III) physicochemical behavior.
This work determined the role of humic acid (HA) and fulvic acid (FA), which was extracted from the natural soil, in the interaction mechanism and microstructure of Eu(III) with pyrolusite (β-MnO2) by using batch experiments and extended X-ray absorption fine structure (EXAFS) techniques. We combined macroscopic and spectroscopic approaches to see the evolution of the interaction mechanism and microstructure of Eu(III) with β-MnO2 in the presence of HA or FA in comparation with that in the absence of HA or FA. The results suggested that Eu(III) interaction with β-MnO2 was obviously affected by the addition of HA or FA. The interaction of Eu(III) with β-MnO2 was enhanced at pH < 6.5 in the presence of HA or FA, while Eu(III) interaction with β-MnO2 was reduced at pH > 6.5 in the presence of HA or FA. The EXAFS fitting results provided a molecular evidence for the findings from the batch experiments. Adsorption of HA or FA onto β-MnO2 greatly modified the microstructure of Eu(III) onto β-MnO2. Only binary surface complexes of Eu(III) can be formed onto β-MnO2 in the absence of HA or FA, while both binary surface complexes and ternary surface complexes of Eu(III) can be simultaneously formed onto β-MnO2 in the presence of HA or FA, which was mainly responsible for the enhanced Eu(III) uptake at low pH values. The results observed in this work are important for the evaluation of physicochemical behavior of long-lived radionuclides (lanthanides and actinides) in the natural soil and water environment.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide