| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147445 | Chemical Engineering Journal | 2014 | 7 Pages |
•High purity BiPO4 was prepared by a hydrothermal route.•Phenol solution is degradable under UV irradiation with BiPO4.•The mineralization of phenol at a low concentration is significant.•The dosage and pH values play a key role in phenol mineralization.
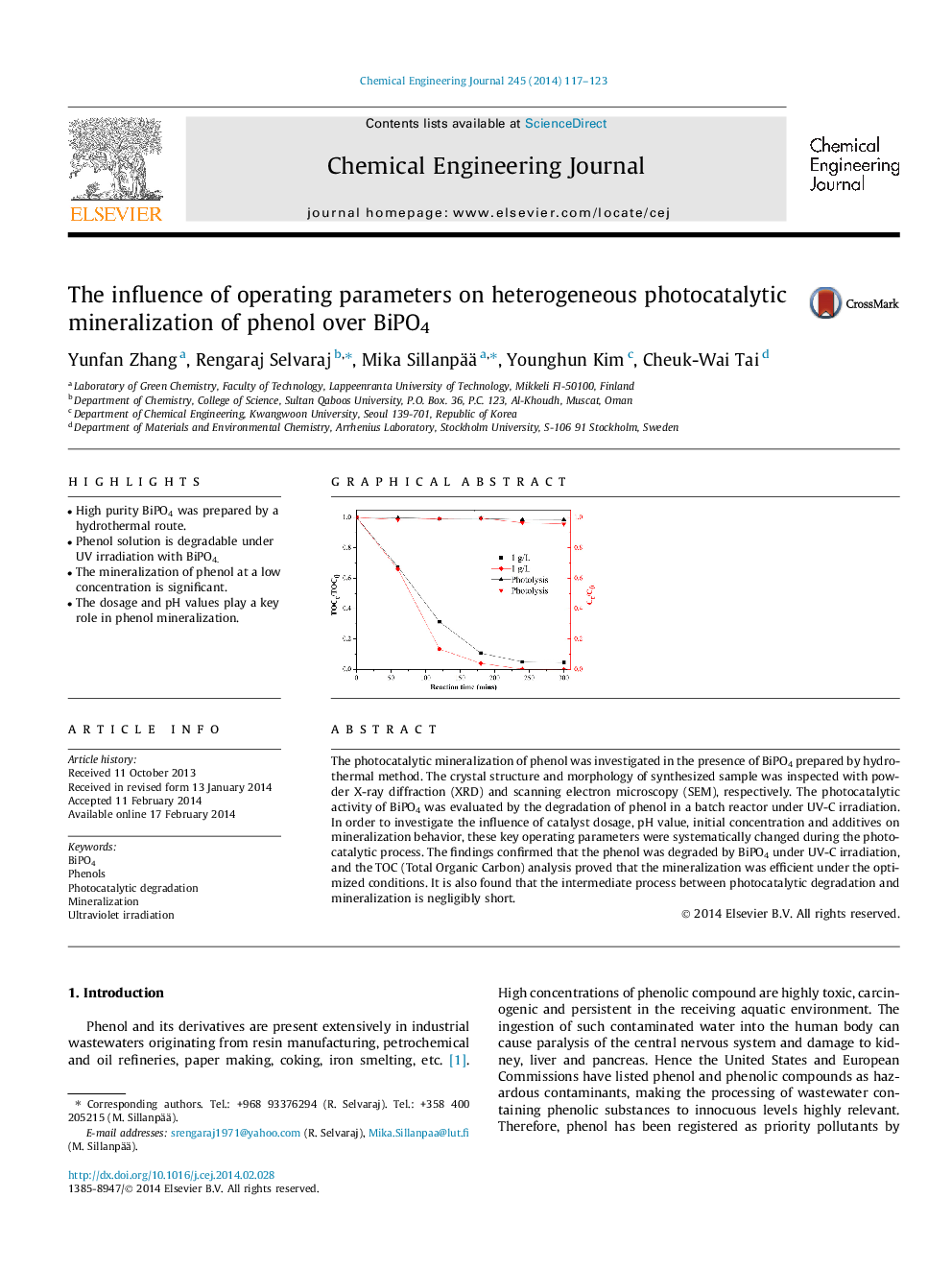
The photocatalytic mineralization of phenol was investigated in the presence of BiPO4 prepared by hydrothermal method. The crystal structure and morphology of synthesized sample was inspected with powder X-ray diffraction (XRD) and scanning electron microscopy (SEM), respectively. The photocatalytic activity of BiPO4 was evaluated by the degradation of phenol in a batch reactor under UV-C irradiation. In order to investigate the influence of catalyst dosage, pH value, initial concentration and additives on mineralization behavior, these key operating parameters were systematically changed during the photocatalytic process. The findings confirmed that the phenol was degraded by BiPO4 under UV-C irradiation, and the TOC (Total Organic Carbon) analysis proved that the mineralization was efficient under the optimized conditions. It is also found that the intermediate process between photocatalytic degradation and mineralization is negligibly short.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide