| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147568 | Chemical Engineering Journal | 2014 | 6 Pages |
•A novel HPMo-based liquid redox process was proposed for H2S removal.•Fuel cell was employed for regeneration of HPMo with co-generation of electricity.•Keggin structure of HPMo showed the best sulfide oxidation and power production.
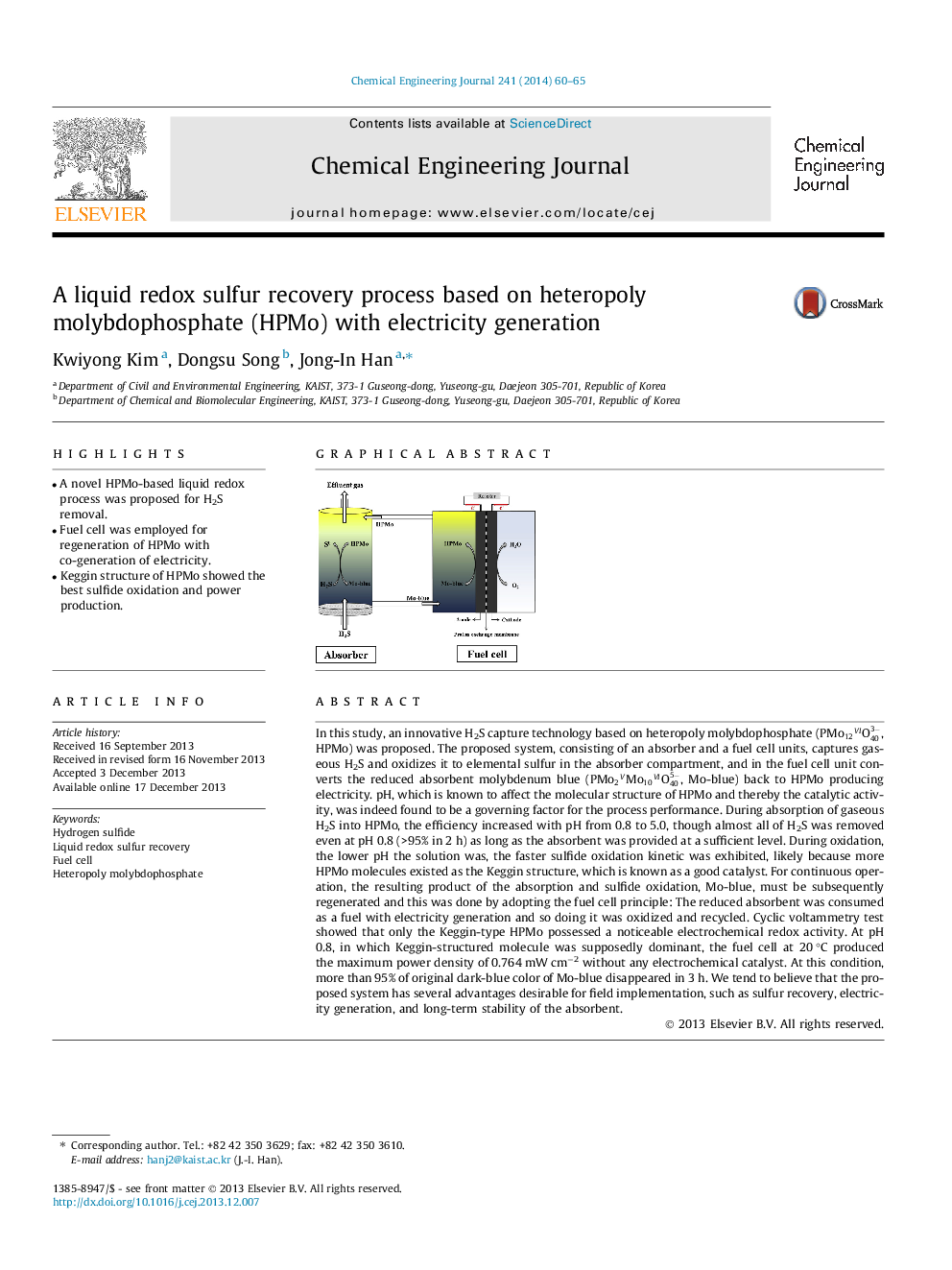
In this study, an innovative H2S capture technology based on heteropoly molybdophosphate (PMo12VIO403-, HPMo) was proposed. The proposed system, consisting of an absorber and a fuel cell units, captures gaseous H2S and oxidizes it to elemental sulfur in the absorber compartment, and in the fuel cell unit converts the reduced absorbent molybdenum blue (PMo2VMo10VIO405-, Mo-blue) back to HPMo producing electricity. pH, which is known to affect the molecular structure of HPMo and thereby the catalytic activity, was indeed found to be a governing factor for the process performance. During absorption of gaseous H2S into HPMo, the efficiency increased with pH from 0.8 to 5.0, though almost all of H2S was removed even at pH 0.8 (>95% in 2 h) as long as the absorbent was provided at a sufficient level. During oxidation, the lower pH the solution was, the faster sulfide oxidation kinetic was exhibited, likely because more HPMo molecules existed as the Keggin structure, which is known as a good catalyst. For continuous operation, the resulting product of the absorption and sulfide oxidation, Mo-blue, must be subsequently regenerated and this was done by adopting the fuel cell principle: The reduced absorbent was consumed as a fuel with electricity generation and so doing it was oxidized and recycled. Cyclic voltammetry test showed that only the Keggin-type HPMo possessed a noticeable electrochemical redox activity. At pH 0.8, in which Keggin-structured molecule was supposedly dominant, the fuel cell at 20 °C produced the maximum power density of 0.764 mW cm−2 without any electrochemical catalyst. At this condition, more than 95% of original dark-blue color of Mo-blue disappeared in 3 h. We tend to believe that the proposed system has several advantages desirable for field implementation, such as sulfur recovery, electricity generation, and long-term stability of the absorbent.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide