| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147675 | Chemical Engineering Journal | 2014 | 8 Pages |
•The T–FC was firstly synthesized by wet-chemical processing and ultrasound irradiation.•This T–FC exhibited higher visible-light photocatalytic and antibacterial activities.•The T–FC can be easily recovered from water using a magnet.•Moreover, the T–FC exhibits excellent photocatalytic stability.
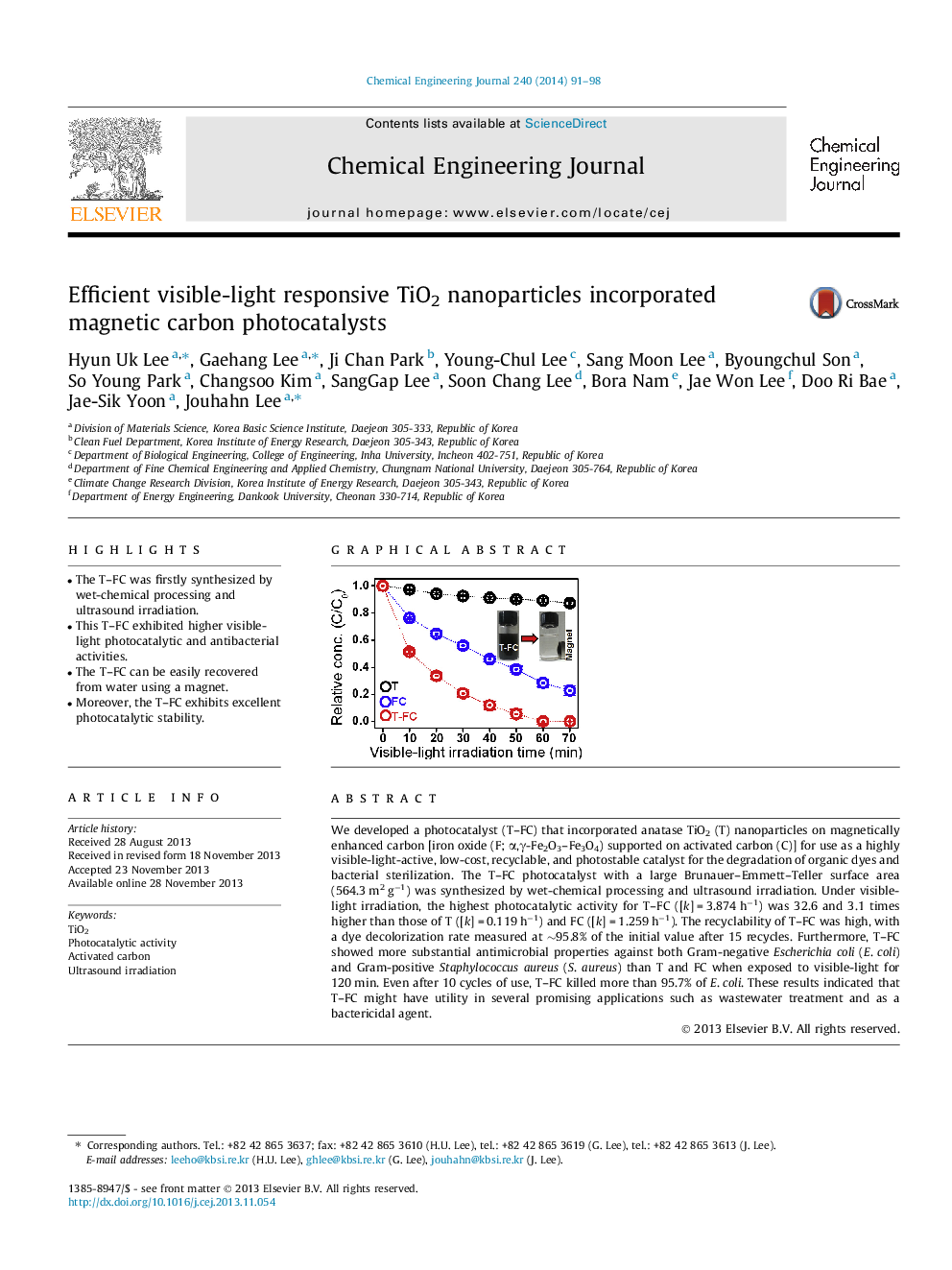
We developed a photocatalyst (T–FC) that incorporated anatase TiO2 (T) nanoparticles on magnetically enhanced carbon [iron oxide (F; α,γ-Fe2O3–Fe3O4) supported on activated carbon (C)] for use as a highly visible-light-active, low-cost, recyclable, and photostable catalyst for the degradation of organic dyes and bacterial sterilization. The T–FC photocatalyst with a large Brunauer–Emmett–Teller surface area (564.3 m2 g−1) was synthesized by wet-chemical processing and ultrasound irradiation. Under visible-light irradiation, the highest photocatalytic activity for T–FC ([k] = 3.874 h−1) was 32.6 and 3.1 times higher than those of T ([k] = 0.119 h−1) and FC ([k] = 1.259 h−1). The recyclability of T–FC was high, with a dye decolorization rate measured at ∼95.8% of the initial value after 15 recycles. Furthermore, T–FC showed more substantial antimicrobial properties against both Gram-negative Escherichia coli (E. coli) and Gram-positive Staphylococcus aureus (S. aureus) than T and FC when exposed to visible-light for 120 min. Even after 10 cycles of use, T–FC killed more than 95.7% of E. coli. These results indicated that T–FC might have utility in several promising applications such as wastewater treatment and as a bactericidal agent.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide