| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147693 | Chemical Engineering Journal | 2014 | 10 Pages |
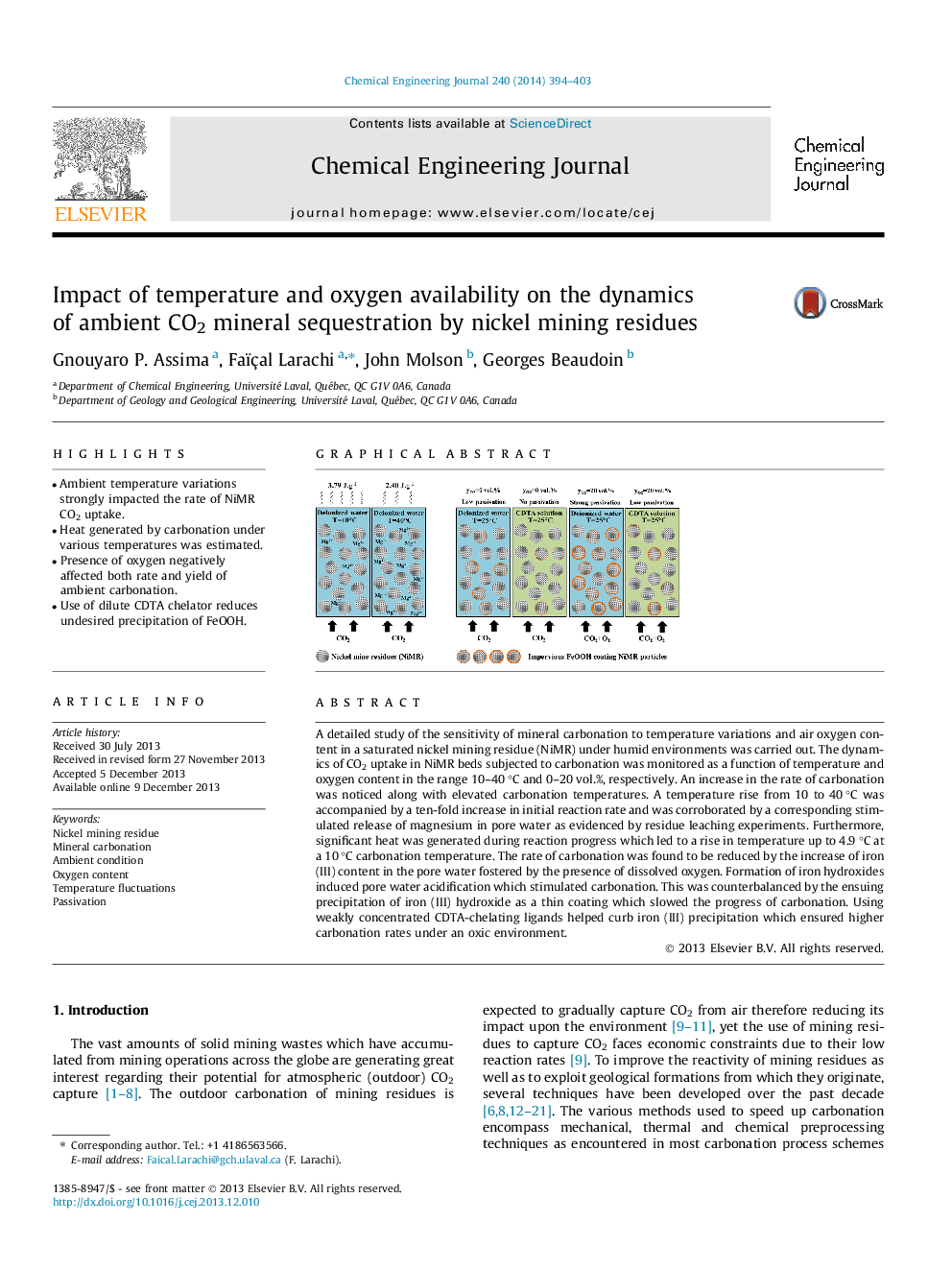
•Ambient temperature variations strongly impacted the rate of NiMR CO2 uptake.•Heat generated by carbonation under various temperatures was estimated.•Presence of oxygen negatively affected both rate and yield of ambient carbonation.•Use of dilute CDTA chelator reduces undesired precipitation of FeOOH.
A detailed study of the sensitivity of mineral carbonation to temperature variations and air oxygen content in a saturated nickel mining residue (NiMR) under humid environments was carried out. The dynamics of CO2 uptake in NiMR beds subjected to carbonation was monitored as a function of temperature and oxygen content in the range 10–40 °C and 0–20 vol.%, respectively. An increase in the rate of carbonation was noticed along with elevated carbonation temperatures. A temperature rise from 10 to 40 °C was accompanied by a ten-fold increase in initial reaction rate and was corroborated by a corresponding stimulated release of magnesium in pore water as evidenced by residue leaching experiments. Furthermore, significant heat was generated during reaction progress which led to a rise in temperature up to 4.9 °C at a 10 °C carbonation temperature. The rate of carbonation was found to be reduced by the increase of iron (III) content in the pore water fostered by the presence of dissolved oxygen. Formation of iron hydroxides induced pore water acidification which stimulated carbonation. This was counterbalanced by the ensuing precipitation of iron (III) hydroxide as a thin coating which slowed the progress of carbonation. Using weakly concentrated CDTA-chelating ligands helped curb iron (III) precipitation which ensured higher carbonation rates under an oxic environment.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide