| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147715 | Chemical Engineering Journal | 2014 | 7 Pages |
•PdCl2–CuCl2–KOAc/AC@Al2O3 catalyst showed a high STY of DMC and a good selectivity.•Higher proportion of Cu+/Cu2+ improved the DMC selectivity and catalyst stability.•The catalytic mechanism of PdCl2–CuCl2–KOAc/AC@Al2O3 catalyst was proposed.
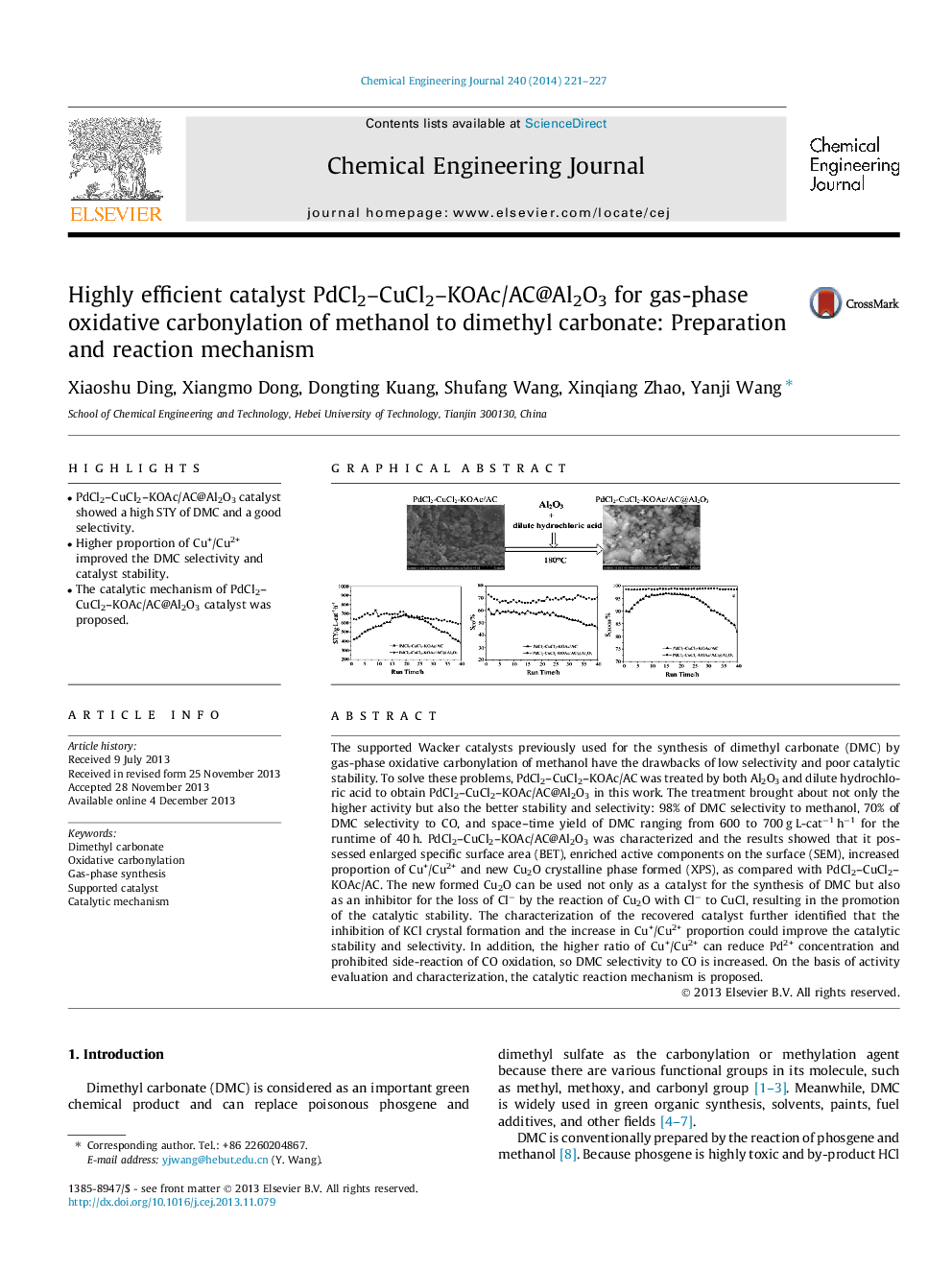
The supported Wacker catalysts previously used for the synthesis of dimethyl carbonate (DMC) by gas-phase oxidative carbonylation of methanol have the drawbacks of low selectivity and poor catalytic stability. To solve these problems, PdCl2–CuCl2–KOAc/AC was treated by both Al2O3 and dilute hydrochloric acid to obtain PdCl2–CuCl2–KOAc/AC@Al2O3 in this work. The treatment brought about not only the higher activity but also the better stability and selectivity: 98% of DMC selectivity to methanol, 70% of DMC selectivity to CO, and space–time yield of DMC ranging from 600 to 700 g L-cat−1 h−1 for the runtime of 40 h. PdCl2–CuCl2–KOAc/AC@Al2O3 was characterized and the results showed that it possessed enlarged specific surface area (BET), enriched active components on the surface (SEM), increased proportion of Cu+/Cu2+ and new Cu2O crystalline phase formed (XPS), as compared with PdCl2–CuCl2–KOAc/AC. The new formed Cu2O can be used not only as a catalyst for the synthesis of DMC but also as an inhibitor for the loss of Cl− by the reaction of Cu2O with Cl− to CuCl, resulting in the promotion of the catalytic stability. The characterization of the recovered catalyst further identified that the inhibition of KCl crystal formation and the increase in Cu+/Cu2+ proportion could improve the catalytic stability and selectivity. In addition, the higher ratio of Cu+/Cu2+ can reduce Pd2+ concentration and prohibited side-reaction of CO oxidation, so DMC selectivity to CO is increased. On the basis of activity evaluation and characterization, the catalytic reaction mechanism is proposed.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide