| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147761 | Chemical Engineering Journal | 2014 | 9 Pages |
•Phase transformation undertakes only during solid-state reaction.•The chemical environment closely regulates the capacity of interlayer cations.•Higher surface basicity allows higher sorption capacity but reduces sorption affinity.•Lower surface basicity reduces the onset potential of oxygen reduction reaction.
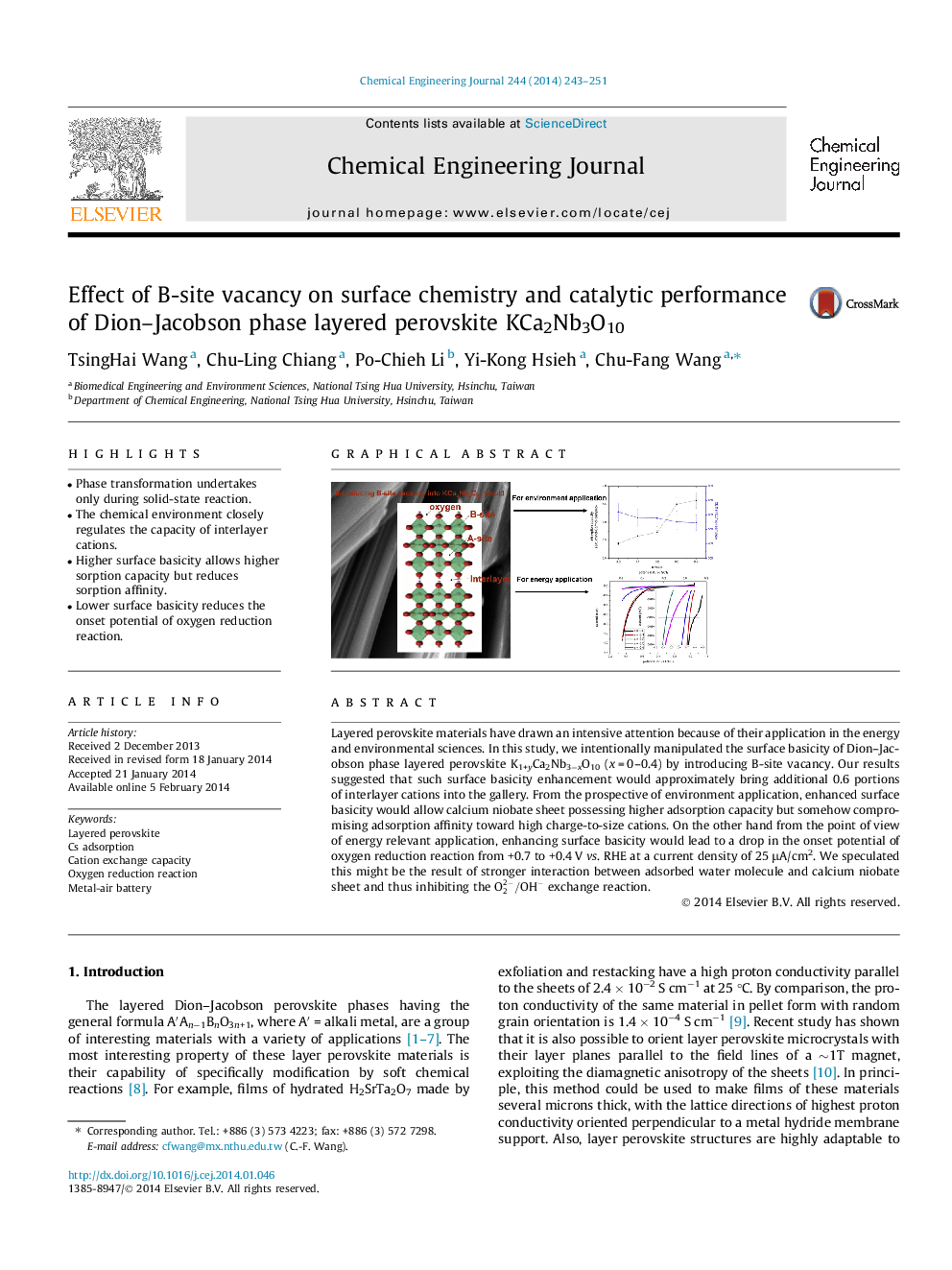
Layered perovskite materials have drawn an intensive attention because of their application in the energy and environmental sciences. In this study, we intentionally manipulated the surface basicity of Dion–Jacobson phase layered perovskite K1+yCa2Nb3−xO10 (x = 0–0.4) by introducing B-site vacancy. Our results suggested that such surface basicity enhancement would approximately bring additional 0.6 portions of interlayer cations into the gallery. From the prospective of environment application, enhanced surface basicity would allow calcium niobate sheet possessing higher adsorption capacity but somehow compromising adsorption affinity toward high charge-to-size cations. On the other hand from the point of view of energy relevant application, enhancing surface basicity would lead to a drop in the onset potential of oxygen reduction reaction from +0.7 to +0.4 V vs. RHE at a current density of 25 μA/cm2. We speculated this might be the result of stronger interaction between adsorbed water molecule and calcium niobate sheet and thus inhibiting the O22-/OH- exchange reaction.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide