| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147812 | Chemical Engineering Journal | 2014 | 10 Pages |
•Magnetic prussian blue/graphene oxide–calcium alginate microbeads were prepared.•Microbeads could be separated rapidly by an external magnetic field.•Adsorption properties for Cs+ of microbeads were studied.•The microbeads present high efficiency and good selectivity to Cs+.•The microbeads are convenient for treatment of cesium-contaminated water and soil.
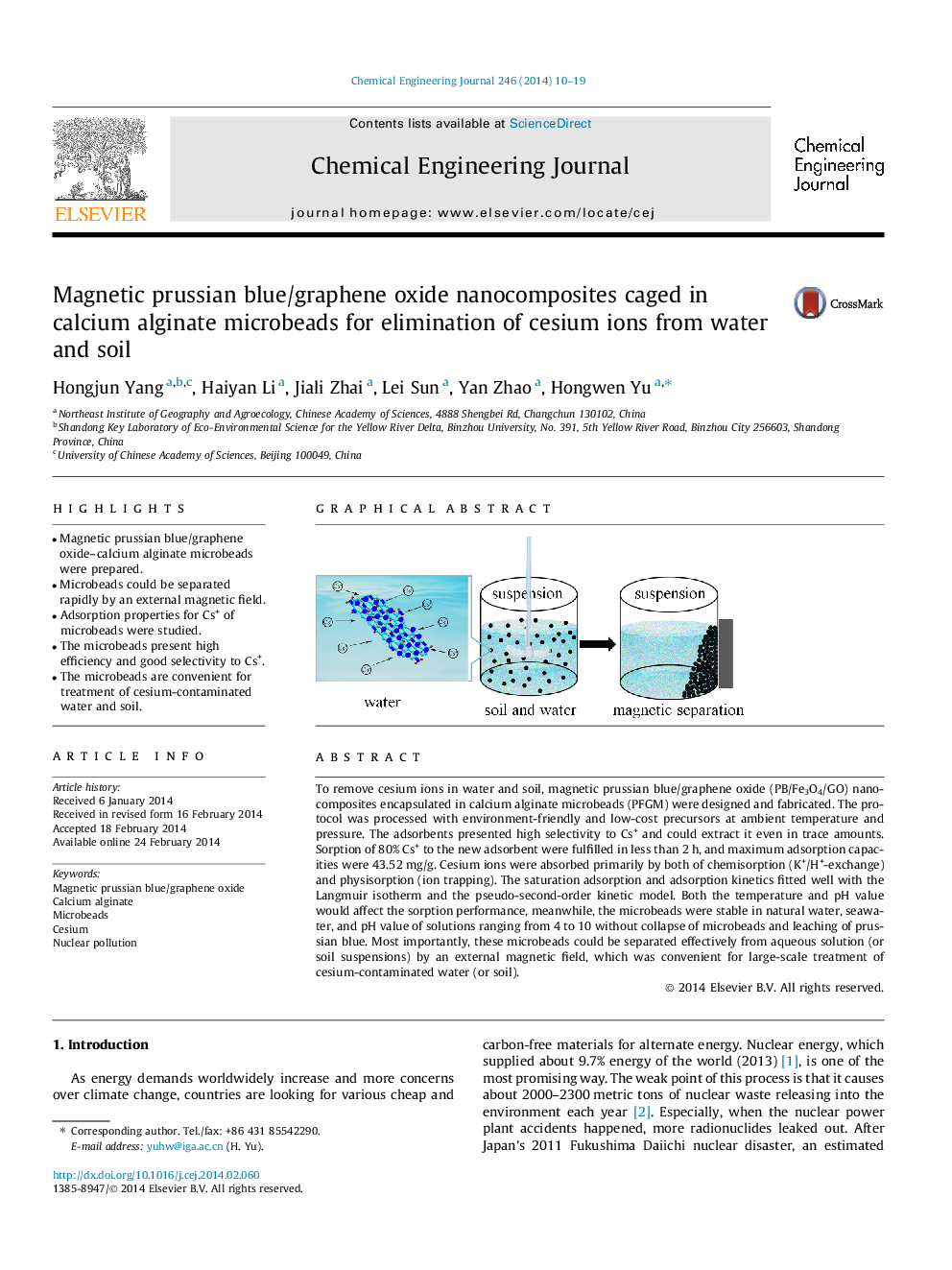
To remove cesium ions in water and soil, magnetic prussian blue/graphene oxide (PB/Fe3O4/GO) nanocomposites encapsulated in calcium alginate microbeads (PFGM) were designed and fabricated. The protocol was processed with environment-friendly and low-cost precursors at ambient temperature and pressure. The adsorbents presented high selectivity to Cs+ and could extract it even in trace amounts. Sorption of 80% Cs+ to the new adsorbent were fulfilled in less than 2 h, and maximum adsorption capacities were 43.52 mg/g. Cesium ions were absorbed primarily by both of chemisorption (K+/H+-exchange) and physisorption (ion trapping). The saturation adsorption and adsorption kinetics fitted well with the Langmuir isotherm and the pseudo-second-order kinetic model. Both the temperature and pH value would affect the sorption performance, meanwhile, the microbeads were stable in natural water, seawater, and pH value of solutions ranging from 4 to 10 without collapse of microbeads and leaching of prussian blue. Most importantly, these microbeads could be separated effectively from aqueous solution (or soil suspensions) by an external magnetic field, which was convenient for large-scale treatment of cesium-contaminated water (or soil).
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide