| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147873 | Chemical Engineering Journal | 2014 | 9 Pages |
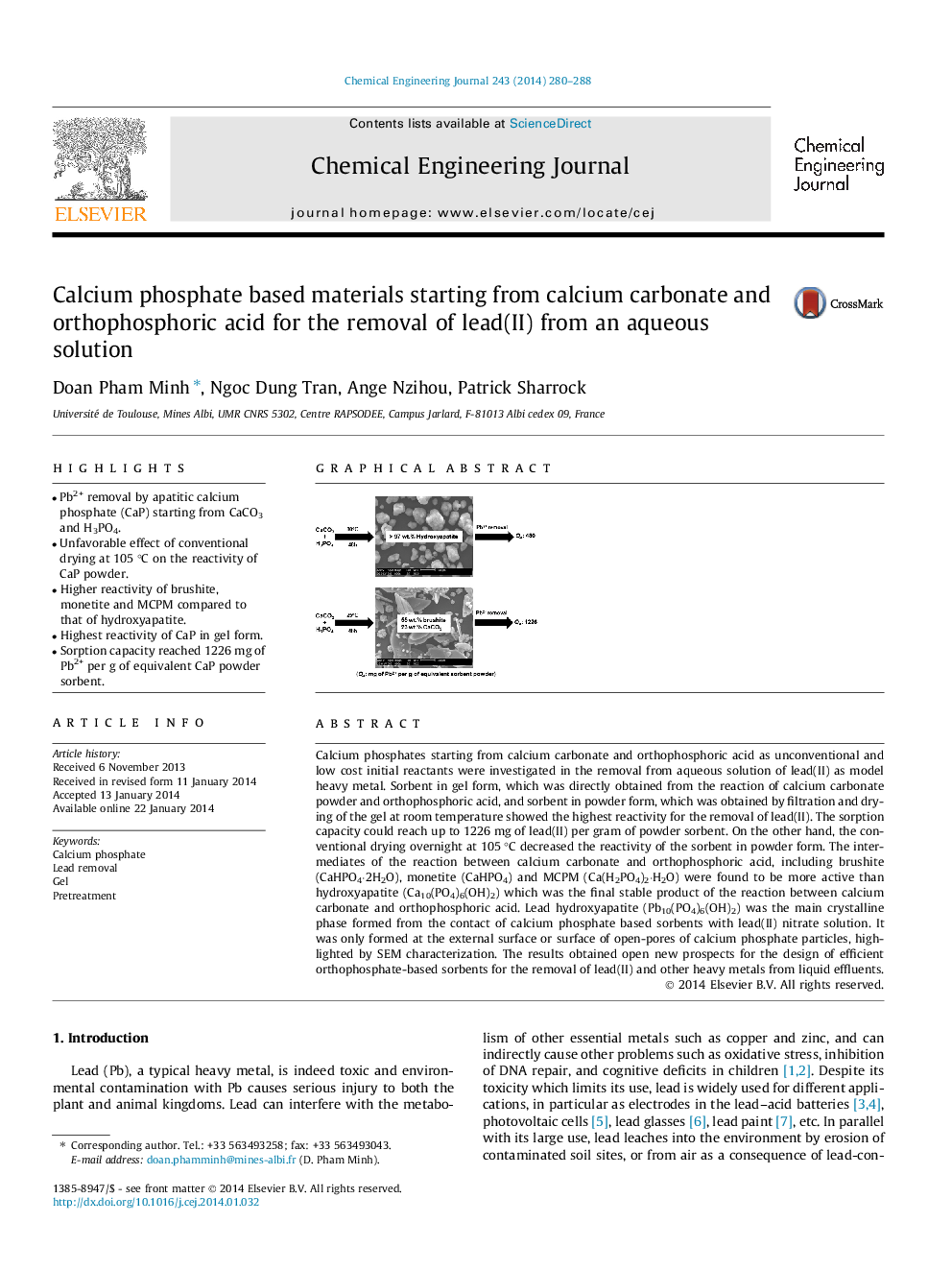
•Pb2+ removal by apatitic calcium phosphate (CaP) starting from CaCO3 and H3PO4.•Unfavorable effect of conventional drying at 105 °C on the reactivity of CaP powder.•Higher reactivity of brushite, monetite and MCPM compared to that of hydroxyapatite.•Highest reactivity of CaP in gel form.•Sorption capacity reached 1226 mg of Pb2+ per g of equivalent CaP powder sorbent.
Calcium phosphates starting from calcium carbonate and orthophosphoric acid as unconventional and low cost initial reactants were investigated in the removal from aqueous solution of lead(II) as model heavy metal. Sorbent in gel form, which was directly obtained from the reaction of calcium carbonate powder and orthophosphoric acid, and sorbent in powder form, which was obtained by filtration and drying of the gel at room temperature showed the highest reactivity for the removal of lead(II). The sorption capacity could reach up to 1226 mg of lead(II) per gram of powder sorbent. On the other hand, the conventional drying overnight at 105 °C decreased the reactivity of the sorbent in powder form. The intermediates of the reaction between calcium carbonate and orthophosphoric acid, including brushite (CaHPO4·2H2O), monetite (CaHPO4) and MCPM (Ca(H2PO4)2·H2O) were found to be more active than hydroxyapatite (Ca10(PO4)6(OH)2) which was the final stable product of the reaction between calcium carbonate and orthophosphoric acid. Lead hydroxyapatite (Pb10(PO4)6(OH)2) was the main crystalline phase formed from the contact of calcium phosphate based sorbents with lead(II) nitrate solution. It was only formed at the external surface or surface of open-pores of calcium phosphate particles, highlighted by SEM characterization. The results obtained open new prospects for the design of efficient orthophosphate-based sorbents for the removal of lead(II) and other heavy metals from liquid effluents.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide