| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147972 | Chemical Engineering Journal | 2014 | 12 Pages |
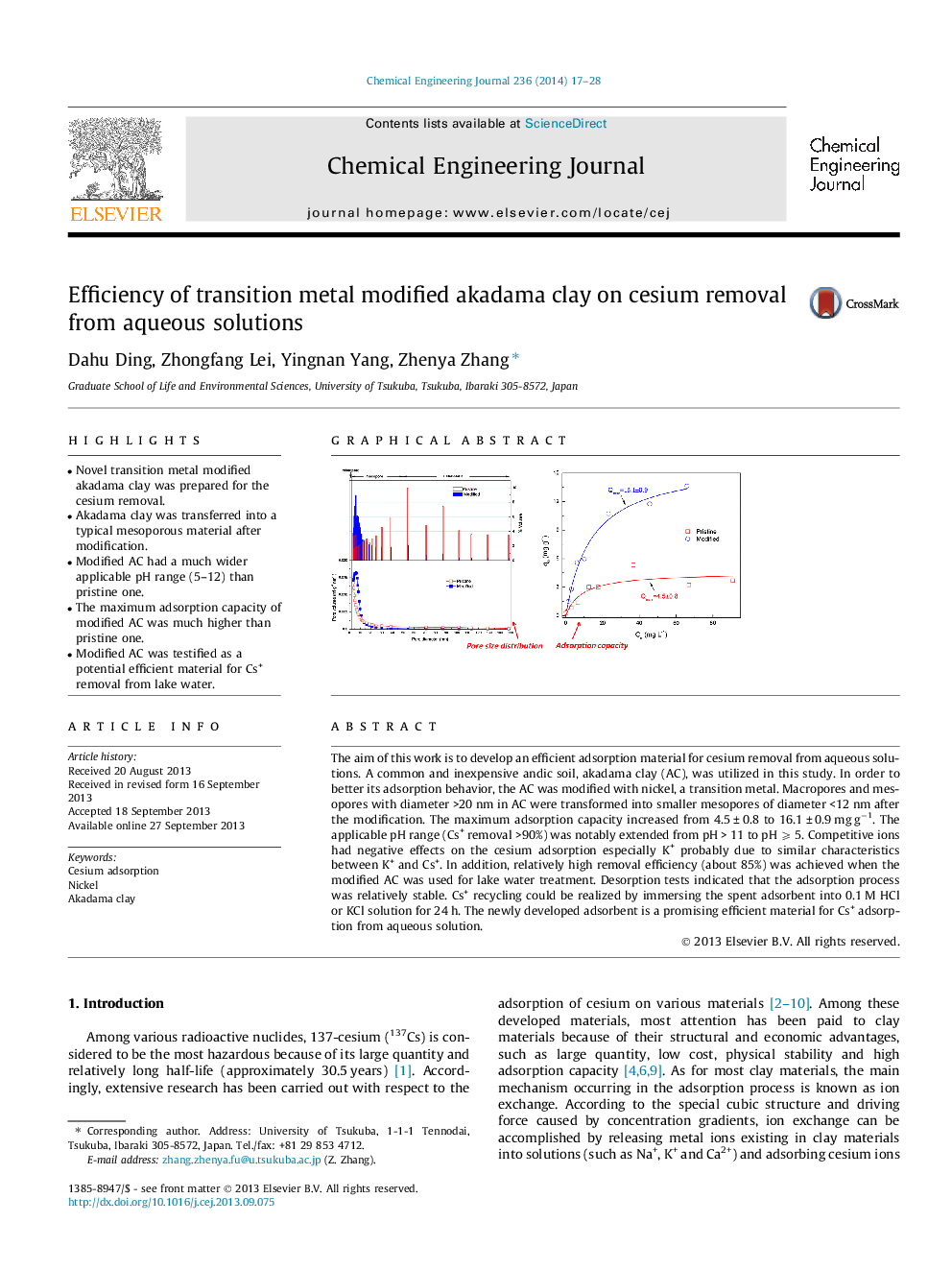
•Novel transition metal modified akadama clay was prepared for the cesium removal.•Akadama clay was transferred into a typical mesoporous material after modification.•Modified AC had a much wider applicable pH range (5–12) than pristine one.•The maximum adsorption capacity of modified AC was much higher than pristine one.•Modified AC was testified as a potential efficient material for Cs+ removal from lake water.
The aim of this work is to develop an efficient adsorption material for cesium removal from aqueous solutions. A common and inexpensive andic soil, akadama clay (AC), was utilized in this study. In order to better its adsorption behavior, the AC was modified with nickel, a transition metal. Macropores and mesopores with diameter >20 nm in AC were transformed into smaller mesopores of diameter <12 nm after the modification. The maximum adsorption capacity increased from 4.5 ± 0.8 to 16.1 ± 0.9 mg g−1. The applicable pH range (Cs+ removal >90%) was notably extended from pH > 11 to pH ⩾ 5. Competitive ions had negative effects on the cesium adsorption especially K+ probably due to similar characteristics between K+ and Cs+. In addition, relatively high removal efficiency (about 85%) was achieved when the modified AC was used for lake water treatment. Desorption tests indicated that the adsorption process was relatively stable. Cs+ recycling could be realized by immersing the spent adsorbent into 0.1 M HCl or KCl solution for 24 h. The newly developed adsorbent is a promising efficient material for Cs+ adsorption from aqueous solution.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide