| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 147990 | Chemical Engineering Journal | 2014 | 9 Pages |
•Synergic removal of AB and Cu (II) was achieved onto HPCR.•Sequential recovery of co-adsorbates was first reported.•Both complex-adsorption and surface complex-interaction were potential mechanisms.•Complex-interaction was the predominant mechanism for the enhanced uptake of Cu (II).•New active sites for Cu (II) coordination were caused by AB on HPCR.
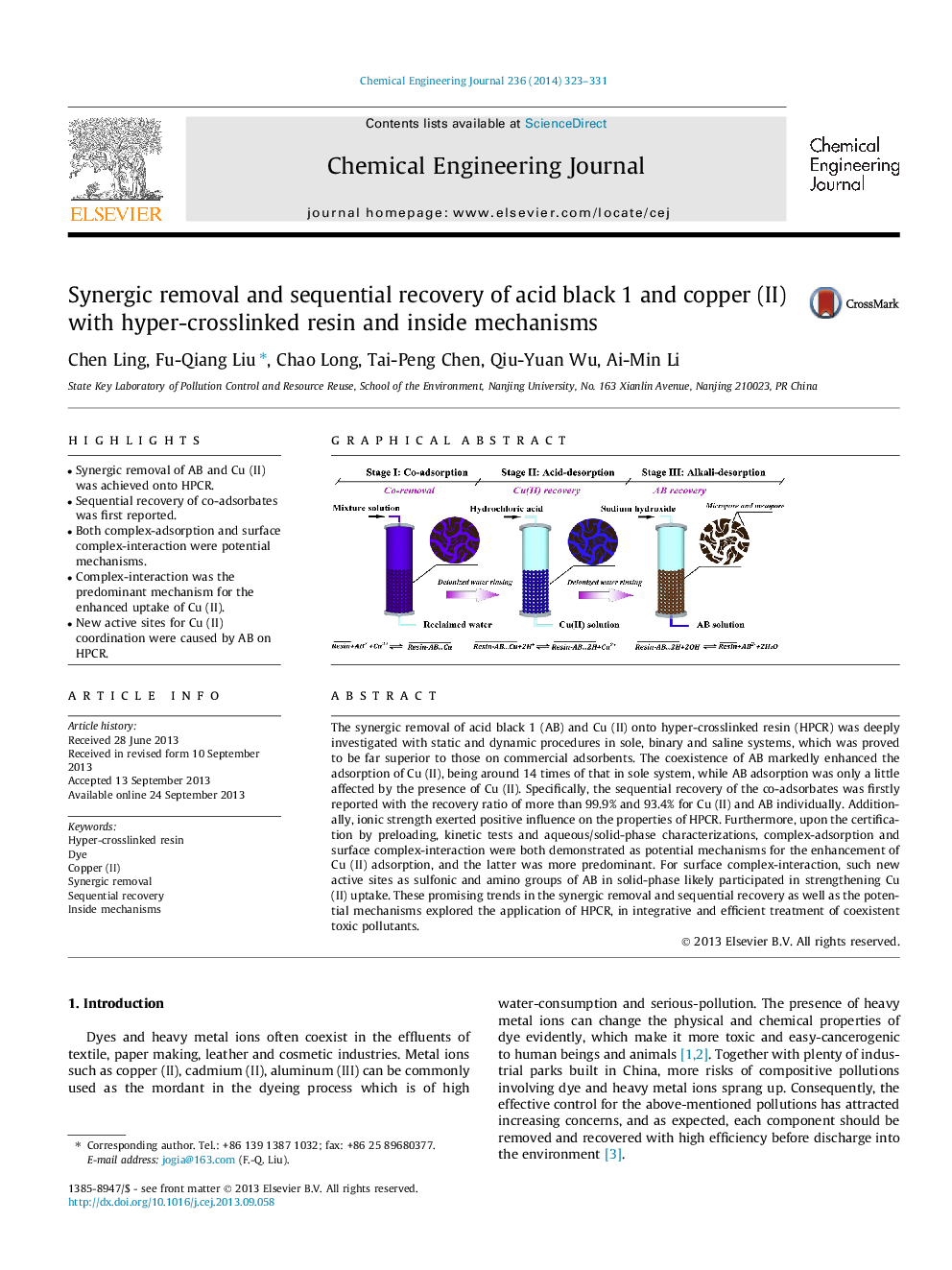
The synergic removal of acid black 1 (AB) and Cu (II) onto hyper-crosslinked resin (HPCR) was deeply investigated with static and dynamic procedures in sole, binary and saline systems, which was proved to be far superior to those on commercial adsorbents. The coexistence of AB markedly enhanced the adsorption of Cu (II), being around 14 times of that in sole system, while AB adsorption was only a little affected by the presence of Cu (II). Specifically, the sequential recovery of the co-adsorbates was firstly reported with the recovery ratio of more than 99.9% and 93.4% for Cu (II) and AB individually. Additionally, ionic strength exerted positive influence on the properties of HPCR. Furthermore, upon the certification by preloading, kinetic tests and aqueous/solid-phase characterizations, complex-adsorption and surface complex-interaction were both demonstrated as potential mechanisms for the enhancement of Cu (II) adsorption, and the latter was more predominant. For surface complex-interaction, such new active sites as sulfonic and amino groups of AB in solid-phase likely participated in strengthening Cu (II) uptake. These promising trends in the synergic removal and sequential recovery as well as the potential mechanisms explored the application of HPCR, in integrative and efficient treatment of coexistent toxic pollutants.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide