| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 148009 | Chemical Engineering Journal | 2014 | 4 Pages |
•A new strategy of urea in sub- and supercritical methanol method was proposed.•The optimal reaction conditions were systematically investigated.•The effects of treating reactor, pressure and water on DMC yield were investigated.•The mechanism of urea in sub- and supercritical methanol system was proposed.
Herein the synthesis of dimethyl carbonate (DMC) from the direct alcoholysis of urea in supercritical methanol system was proposed. The effects of different reaction conditions, such as reaction temperature, reaction time, the molar ratio of methanol/urea, reaction pressure, the volume of reaction solution, the treating reactor and the amount of water on DMC yield were systematically investigated. The experimental results indicated that the optimal reaction conditions were reaction temperature of 538 K, time of 2 h, the molar ratio of methanol/urea of 14, and the reactor loading of 285 μL, respectively. And the DMC yield was 98% under the optimal reaction condition.
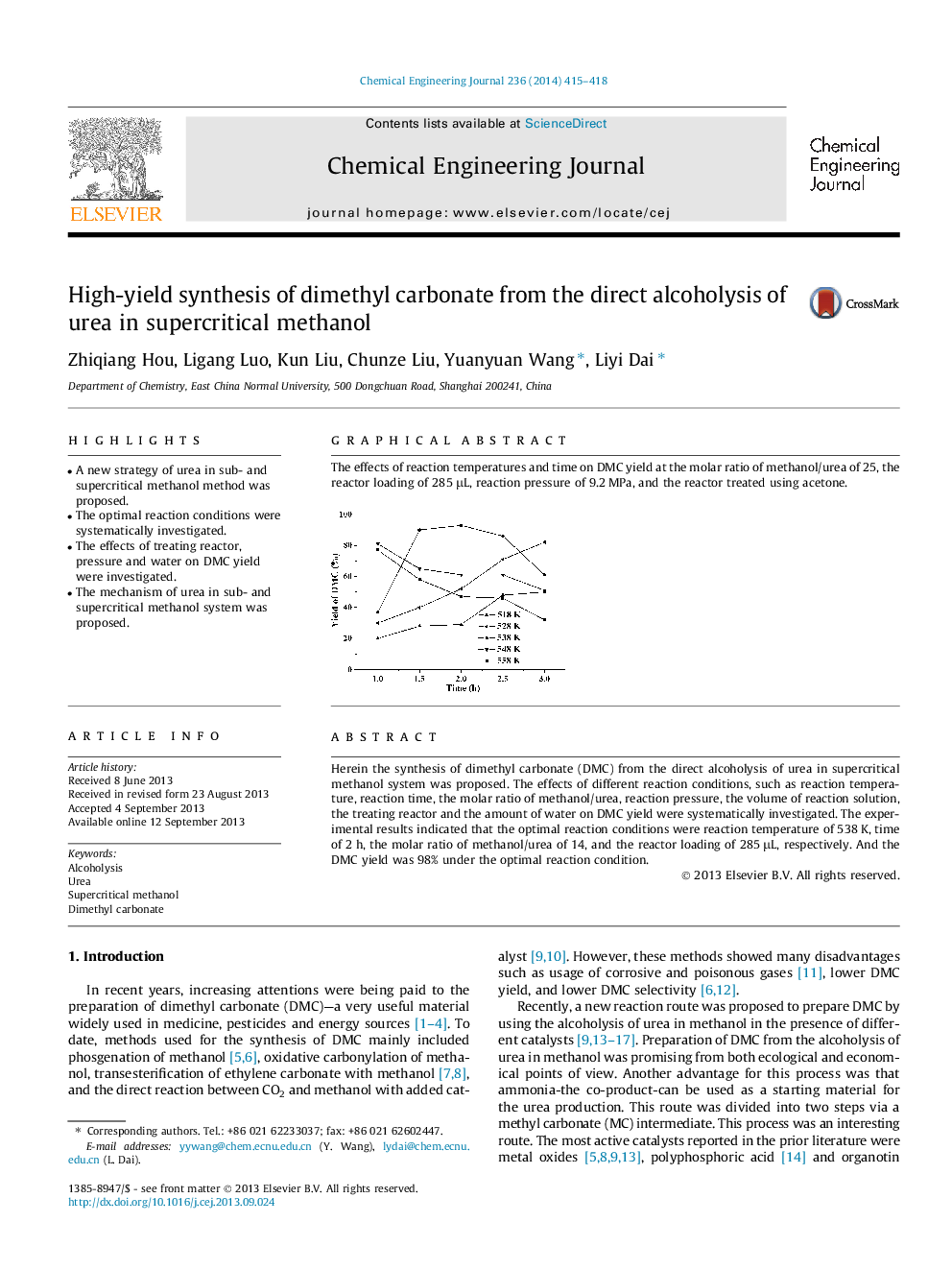
Graphical abstractThe effects of reaction temperatures and time on DMC yield at the molar ratio of methanol/urea of 25, the reactor loading of 285 μL, reaction pressure of 9.2 MPa, and the reactor treated using acetone.Figure optionsDownload full-size imageDownload as PowerPoint slide