| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 148089 | Chemical Engineering Journal | 2013 | 7 Pages |
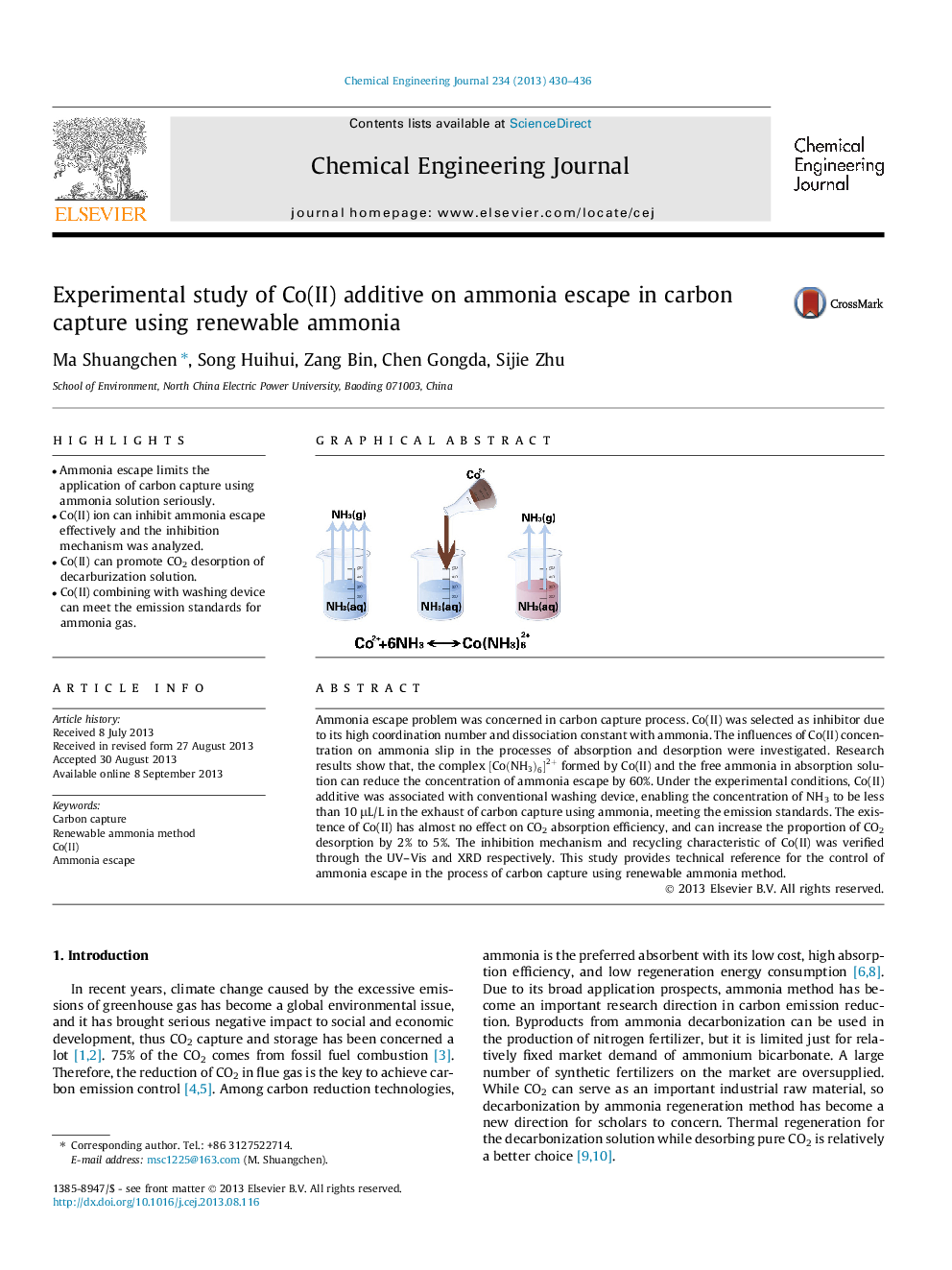
•Ammonia escape limits the application of carbon capture using ammonia solution seriously.•Co(II) ion can inhibit ammonia escape effectively and the inhibition mechanism was analyzed.•Co(II) can promote CO2 desorption of decarburization solution.•Co(II) combining with washing device can meet the emission standards for ammonia gas.
Ammonia escape problem was concerned in carbon capture process. Co(II) was selected as inhibitor due to its high coordination number and dissociation constant with ammonia. The influences of Co(II) concentration on ammonia slip in the processes of absorption and desorption were investigated. Research results show that, the complex [Co(NH3)6]2+[Co(NH3)6]2+ formed by Co(II) and the free ammonia in absorption solution can reduce the concentration of ammonia escape by 60%. Under the experimental conditions, Co(II) additive was associated with conventional washing device, enabling the concentration of NH3 to be less than 10 μL/L in the exhaust of carbon capture using ammonia, meeting the emission standards. The existence of Co(II) has almost no effect on CO2 absorption efficiency, and can increase the proportion of CO2 desorption by 2% to 5%. The inhibition mechanism and recycling characteristic of Co(II) was verified through the UV–Vis and XRD respectively. This study provides technical reference for the control of ammonia escape in the process of carbon capture using renewable ammonia method.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide