| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 148138 | Chemical Engineering Journal | 2013 | 9 Pages |
•TNTs showed large adsorption capacity for Cr(III) with mechanism of ion-exchange.•Mutual promotion on adsorption of Cr(III) and Cr(VI) was found in wide pH ranges.•Formation of Cr(III)OCr(VI) linkages resulted in double-layer adsorption.•Cr(III) rearranged from second layer to TNTs at unsaturated adsorption.
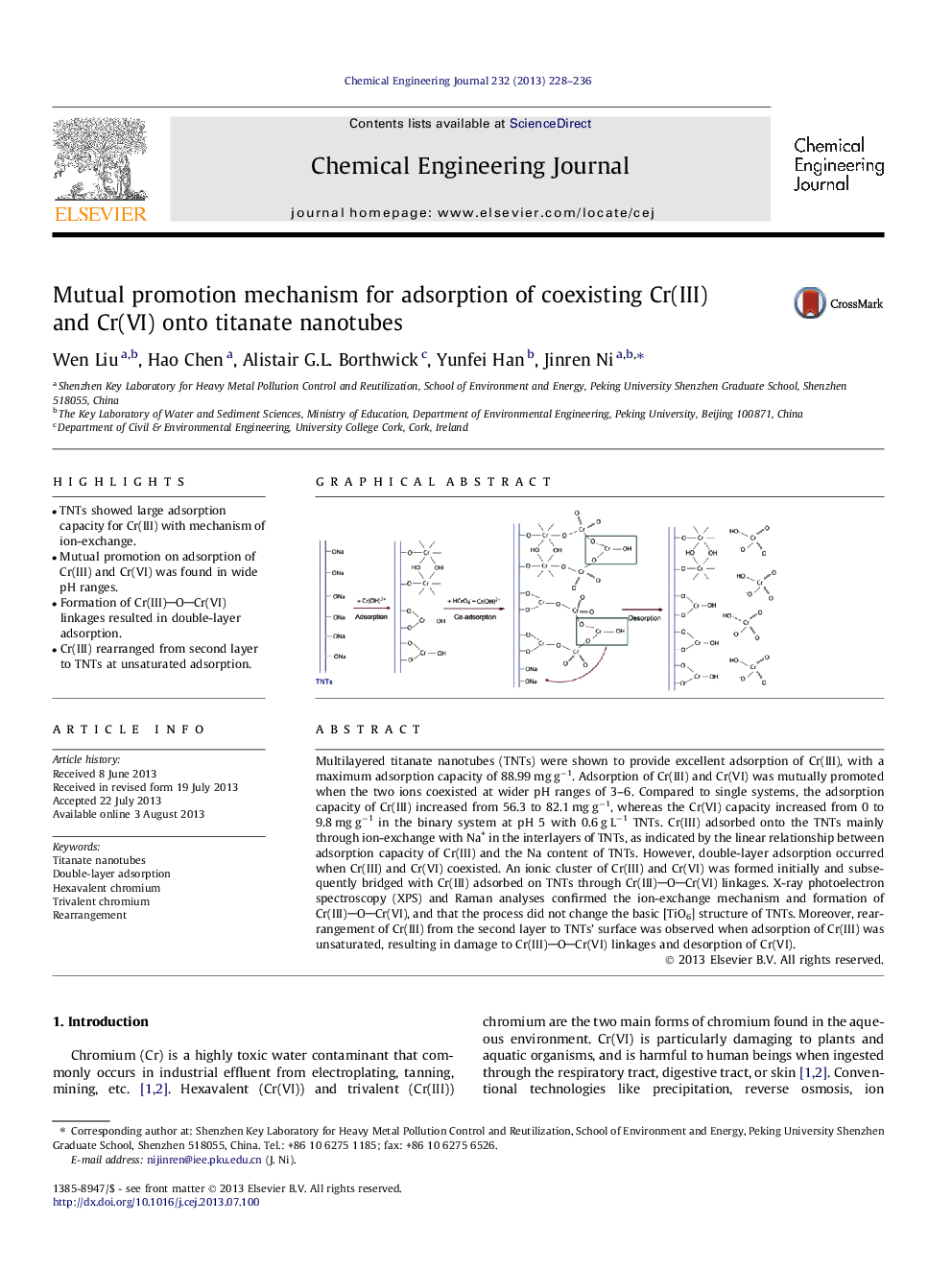
Multilayered titanate nanotubes (TNTs) were shown to provide excellent adsorption of Cr(III), with a maximum adsorption capacity of 88.99 mg g−1. Adsorption of Cr(III) and Cr(VI) was mutually promoted when the two ions coexisted at wider pH ranges of 3–6. Compared to single systems, the adsorption capacity of Cr(III) increased from 56.3 to 82.1 mg g−1, whereas the Cr(VI) capacity increased from 0 to 9.8 mg g−1 in the binary system at pH 5 with 0.6 g L−1 TNTs. Cr(III) adsorbed onto the TNTs mainly through ion-exchange with Na+ in the interlayers of TNTs, as indicated by the linear relationship between adsorption capacity of Cr(III) and the Na content of TNTs. However, double-layer adsorption occurred when Cr(III) and Cr(VI) coexisted. An ionic cluster of Cr(III) and Cr(VI) was formed initially and subsequently bridged with Cr(III) adsorbed on TNTs through Cr(III)OCr(VI) linkages. X-ray photoelectron spectroscopy (XPS) and Raman analyses confirmed the ion-exchange mechanism and formation of Cr(III)OCr(VI), and that the process did not change the basic [TiO6] structure of TNTs. Moreover, rearrangement of Cr(III) from the second layer to TNTs’ surface was observed when adsorption of Cr(III) was unsaturated, resulting in damage to Cr(III)OCr(VI) linkages and desorption of Cr(VI).
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide