| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 148332 | Chemical Engineering Journal | 2014 | 9 Pages |
•Amidoxime-functionalized silica coated Fe3O4 was synthesized and characterized.•Fe3O4@SiO2-AO exhibited fast and efficient sorption for U(VI).•Fe3O4@SiO2-AO could be easily separated from aqueous solutions with a magnet.•Fe3O4@SiO2-AO was resistant to acid corrosion.•U(VI)-loaded Fe3O4@SiO2-AO could be efficiently regenerated and reused.
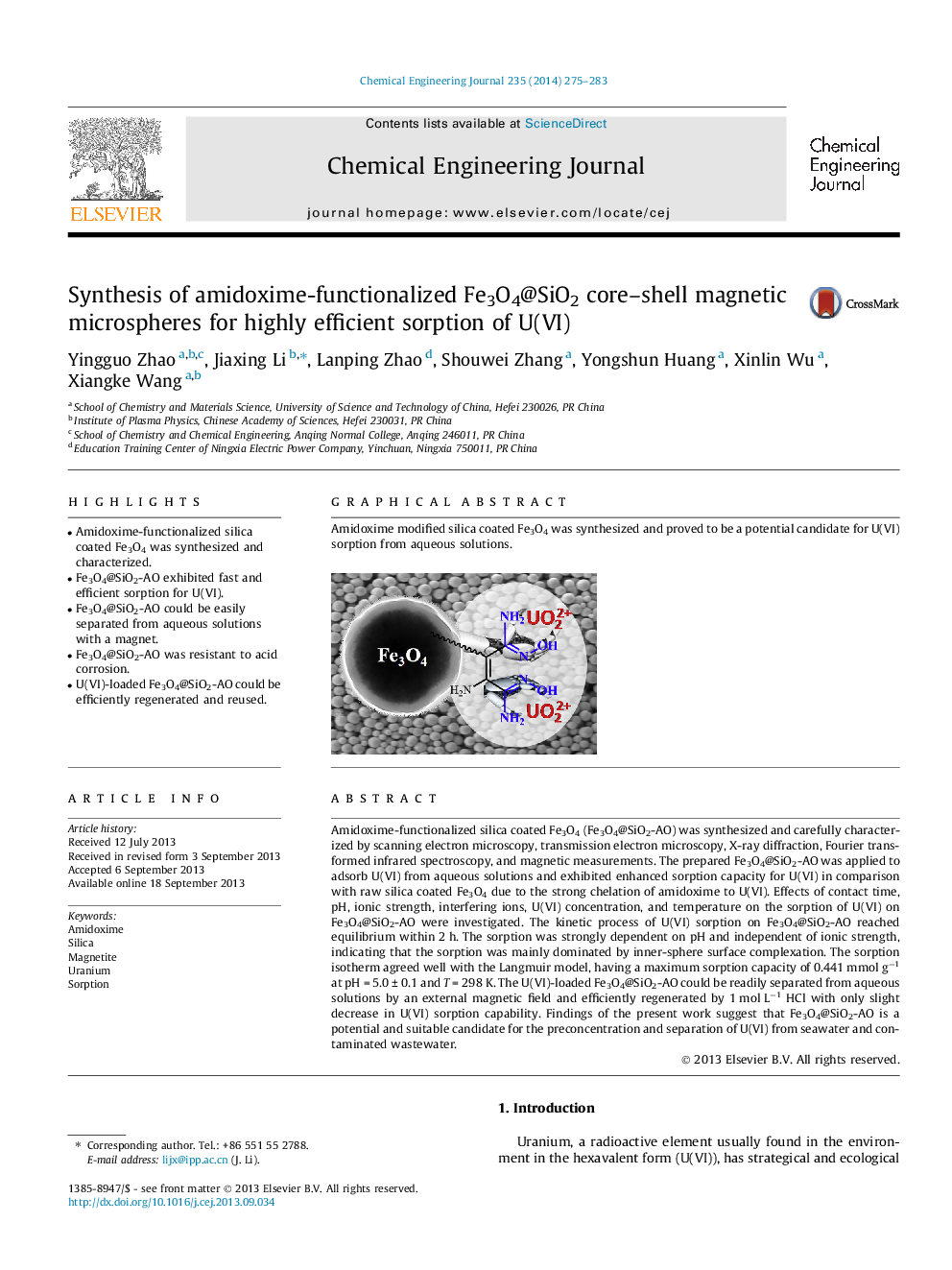
Amidoxime-functionalized silica coated Fe3O4 (Fe3O4@SiO2-AO) was synthesized and carefully characterized by scanning electron microscopy, transmission electron microscopy, X-ray diffraction, Fourier transformed infrared spectroscopy, and magnetic measurements. The prepared Fe3O4@SiO2-AO was applied to adsorb U(VI) from aqueous solutions and exhibited enhanced sorption capacity for U(VI) in comparison with raw silica coated Fe3O4 due to the strong chelation of amidoxime to U(VI). Effects of contact time, pH, ionic strength, interfering ions, U(VI) concentration, and temperature on the sorption of U(VI) on Fe3O4@SiO2-AO were investigated. The kinetic process of U(VI) sorption on Fe3O4@SiO2-AO reached equilibrium within 2 h. The sorption was strongly dependent on pH and independent of ionic strength, indicating that the sorption was mainly dominated by inner-sphere surface complexation. The sorption isotherm agreed well with the Langmuir model, having a maximum sorption capacity of 0.441 mmol g−1 at pH = 5.0 ± 0.1 and T = 298 K. The U(VI)-loaded Fe3O4@SiO2-AO could be readily separated from aqueous solutions by an external magnetic field and efficiently regenerated by 1 mol L−1 HCl with only slight decrease in U(VI) sorption capability. Findings of the present work suggest that Fe3O4@SiO2-AO is a potential and suitable candidate for the preconcentration and separation of U(VI) from seawater and contaminated wastewater.
Graphical abstractAmidoxime modified silica coated Fe3O4 was synthesized and proved to be a potential candidate for U(VI) sorption from aqueous solutions.Figure optionsDownload full-size imageDownload as PowerPoint slide