| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 148386 | Chemical Engineering Journal | 2013 | 10 Pages |
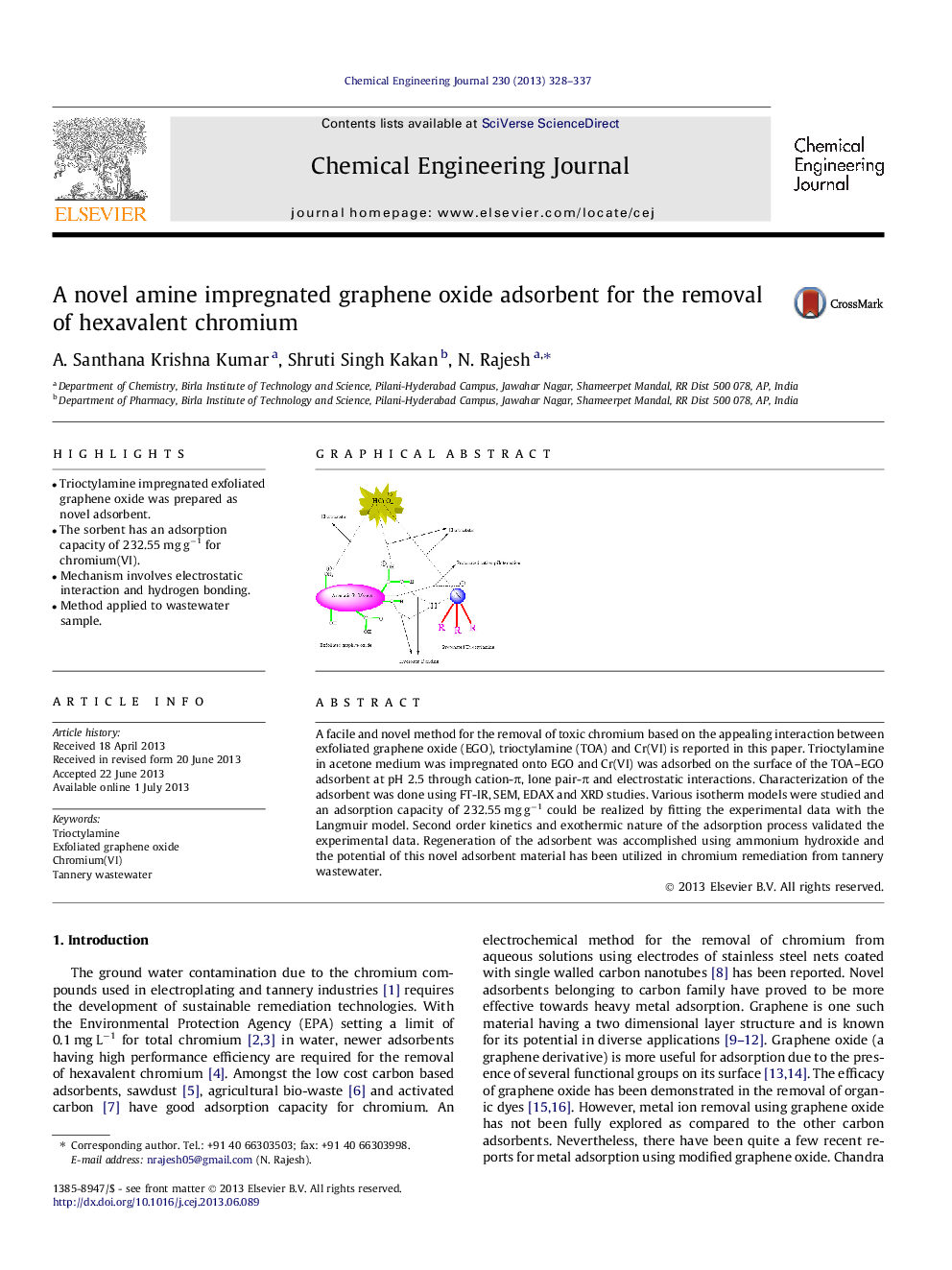
•Trioctylamine impregnated exfoliated graphene oxide was prepared as novel adsorbent.•The sorbent has an adsorption capacity of 232.55 mg g−1 for chromium(VI).•Mechanism involves electrostatic interaction and hydrogen bonding.•Method applied to wastewater sample.
A facile and novel method for the removal of toxic chromium based on the appealing interaction between exfoliated graphene oxide (EGO), trioctylamine (TOA) and Cr(VI) is reported in this paper. Trioctylamine in acetone medium was impregnated onto EGO and Cr(VI) was adsorbed on the surface of the TOA–EGO adsorbent at pH 2.5 through cation-π, lone pair-π and electrostatic interactions. Characterization of the adsorbent was done using FT-IR, SEM, EDAX and XRD studies. Various isotherm models were studied and an adsorption capacity of 232.55 mg g−1 could be realized by fitting the experimental data with the Langmuir model. Second order kinetics and exothermic nature of the adsorption process validated the experimental data. Regeneration of the adsorbent was accomplished using ammonium hydroxide and the potential of this novel adsorbent material has been utilized in chromium remediation from tannery wastewater.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide