| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 148416 | Chemical Engineering Journal | 2013 | 9 Pages |
•Plasmonic Ag@AgBr was intercalated in the layered space of K4Nb6O17.•Ag@AgBr greatly increased visible light absorption for K4Nb6O17.•The plasmonic photocatalysts exhibited enhanced activity for the degradation of RhB.
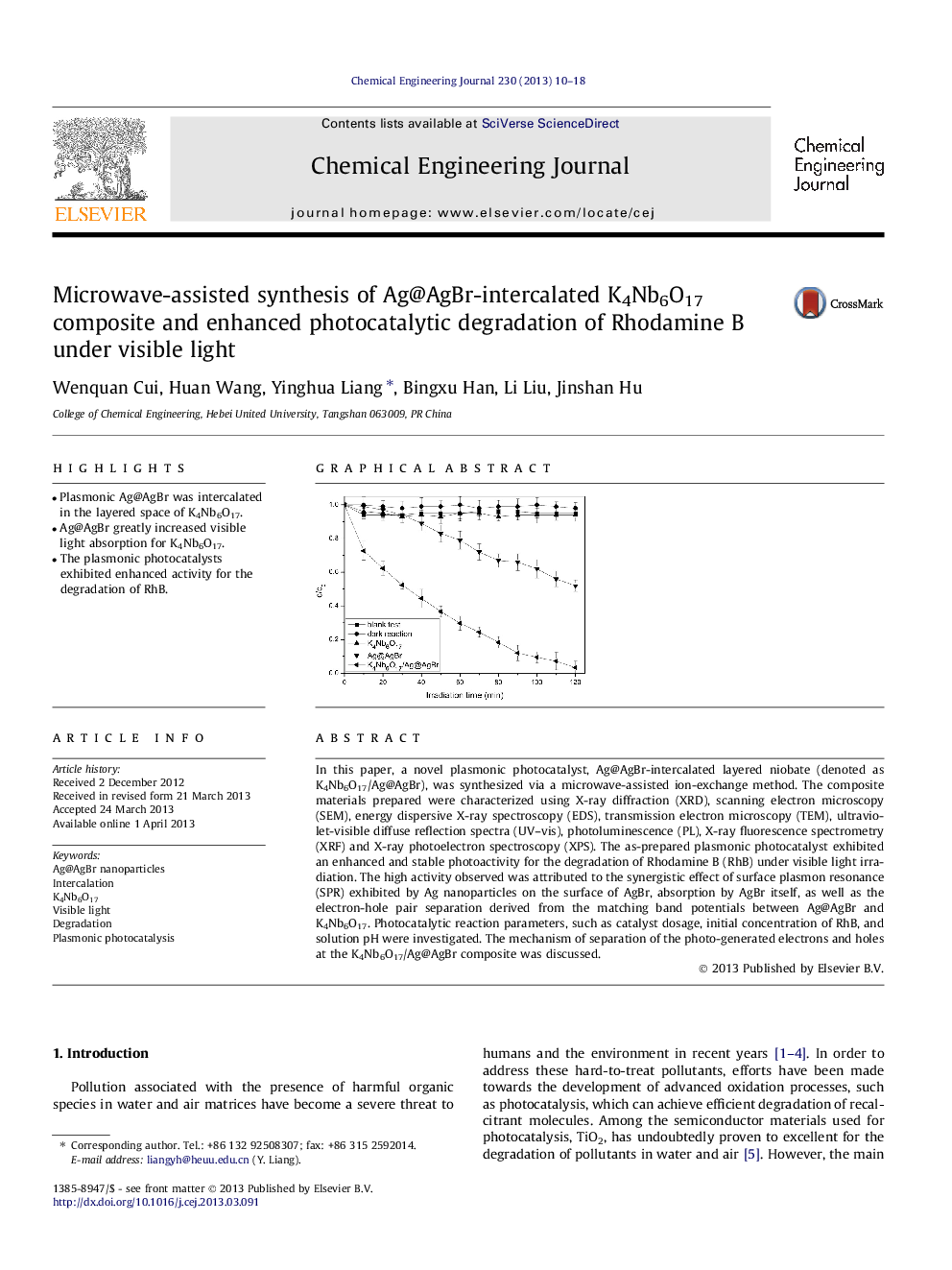
In this paper, a novel plasmonic photocatalyst, Ag@AgBr-intercalated layered niobate (denoted as K4Nb6O17/Ag@AgBr), was synthesized via a microwave-assisted ion-exchange method. The composite materials prepared were characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS), transmission electron microscopy (TEM), ultraviolet-visible diffuse reflection spectra (UV–vis), photoluminescence (PL), X-ray fluorescence spectrometry (XRF) and X-ray photoelectron spectroscopy (XPS). The as-prepared plasmonic photocatalyst exhibited an enhanced and stable photoactivity for the degradation of Rhodamine B (RhB) under visible light irradiation. The high activity observed was attributed to the synergistic effect of surface plasmon resonance (SPR) exhibited by Ag nanoparticles on the surface of AgBr, absorption by AgBr itself, as well as the electron-hole pair separation derived from the matching band potentials between Ag@AgBr and K4Nb6O17. Photocatalytic reaction parameters, such as catalyst dosage, initial concentration of RhB, and solution pH were investigated. The mechanism of separation of the photo-generated electrons and holes at the K4Nb6O17/Ag@AgBr composite was discussed.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide