| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 148450 | Chemical Engineering Journal | 2013 | 8 Pages |
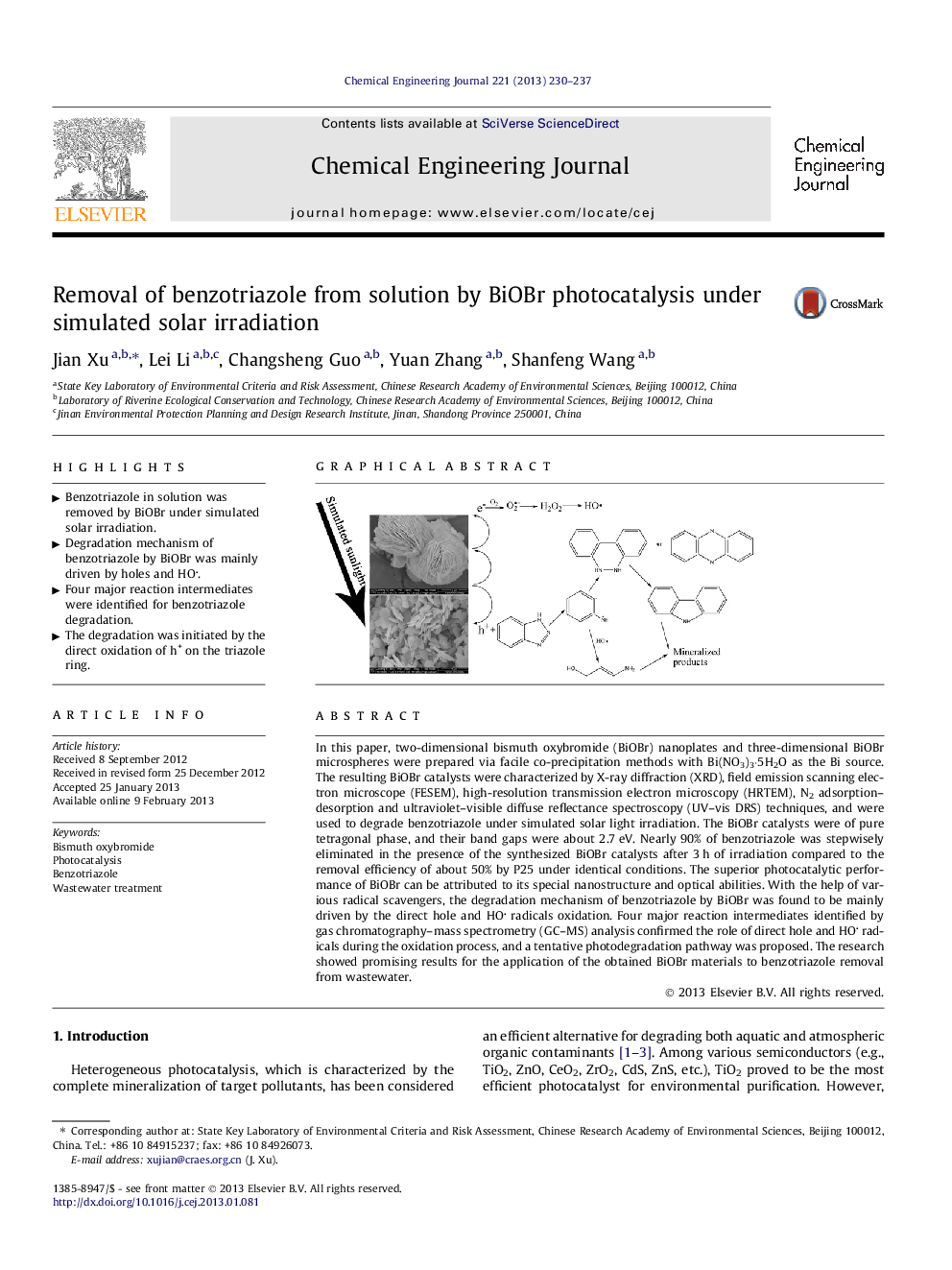
In this paper, two-dimensional bismuth oxybromide (BiOBr) nanoplates and three-dimensional BiOBr microspheres were prepared via facile co-precipitation methods with Bi(NO3)3·5H2O as the Bi source. The resulting BiOBr catalysts were characterized by X-ray diffraction (XRD), field emission scanning electron microscope (FESEM), high-resolution transmission electron microscopy (HRTEM), N2 adsorption–desorption and ultraviolet–visible diffuse reflectance spectroscopy (UV–vis DRS) techniques, and were used to degrade benzotriazole under simulated solar light irradiation. The BiOBr catalysts were of pure tetragonal phase, and their band gaps were about 2.7 eV. Nearly 90% of benzotriazole was stepwisely eliminated in the presence of the synthesized BiOBr catalysts after 3 h of irradiation compared to the removal efficiency of about 50% by P25 under identical conditions. The superior photocatalytic performance of BiOBr can be attributed to its special nanostructure and optical abilities. With the help of various radical scavengers, the degradation mechanism of benzotriazole by BiOBr was found to be mainly driven by the direct hole and HO radicals oxidation. Four major reaction intermediates identified by gas chromatography–mass spectrometry (GC–MS) analysis confirmed the role of direct hole and HO radicals during the oxidation process, and a tentative photodegradation pathway was proposed. The research showed promising results for the application of the obtained BiOBr materials to benzotriazole removal from wastewater.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights• Benzotriazole in solution was removed by BiOBr under simulated solar irradiation. • Degradation mechanism of benzotriazole by BiOBr was mainly driven by holes and HO. • Four major reaction intermediates were identified for benzotriazole degradation. • The degradation was initiated by the direct oxidation of h+ on the triazole ring.