| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 148474 | Chemical Engineering Journal | 2013 | 16 Pages |
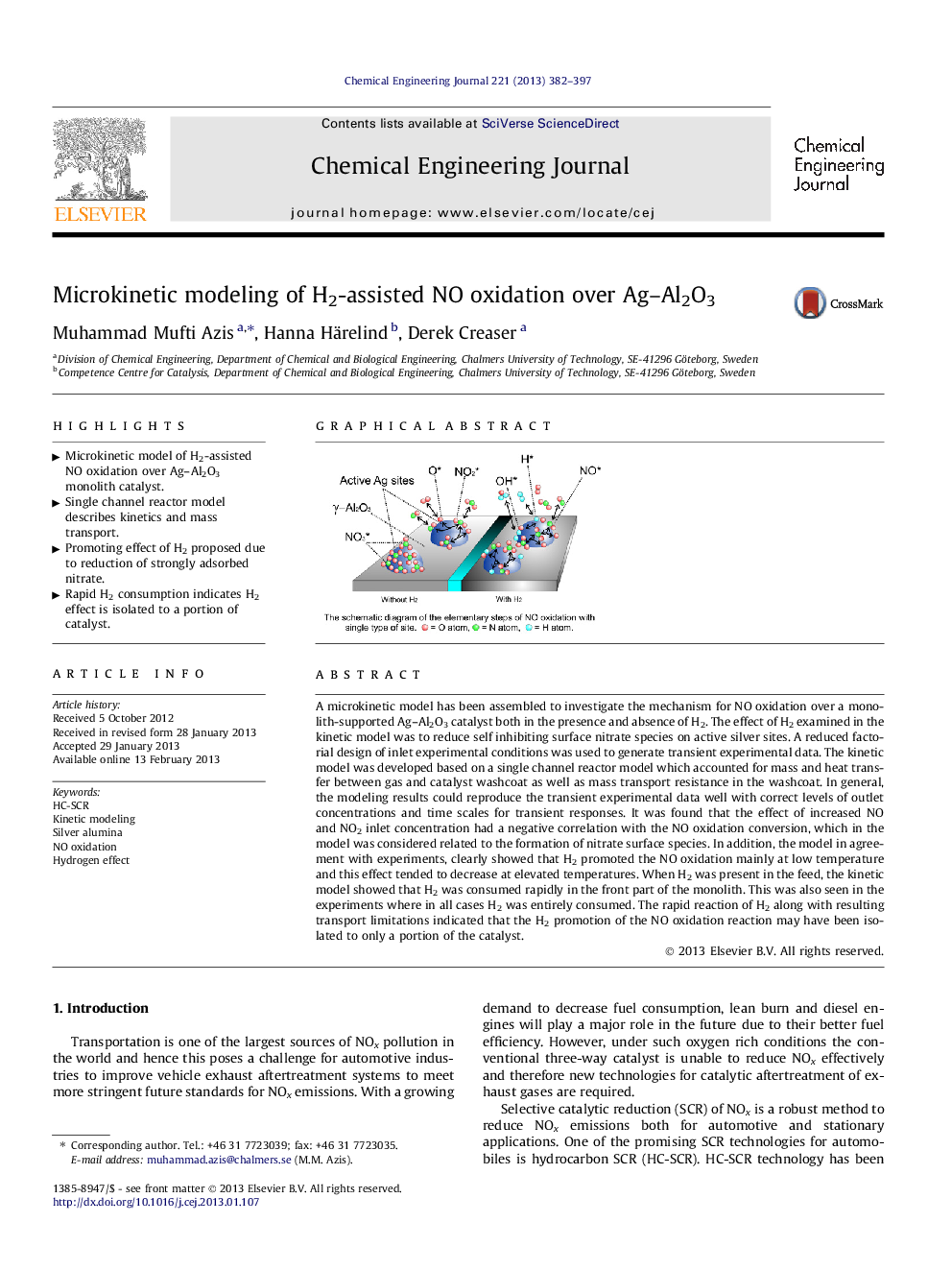
A microkinetic model has been assembled to investigate the mechanism for NO oxidation over a monolith-supported Ag–Al2O3 catalyst both in the presence and absence of H2. The effect of H2 examined in the kinetic model was to reduce self inhibiting surface nitrate species on active silver sites. A reduced factorial design of inlet experimental conditions was used to generate transient experimental data. The kinetic model was developed based on a single channel reactor model which accounted for mass and heat transfer between gas and catalyst washcoat as well as mass transport resistance in the washcoat. In general, the modeling results could reproduce the transient experimental data well with correct levels of outlet concentrations and time scales for transient responses. It was found that the effect of increased NO and NO2 inlet concentration had a negative correlation with the NO oxidation conversion, which in the model was considered related to the formation of nitrate surface species. In addition, the model in agreement with experiments, clearly showed that H2 promoted the NO oxidation mainly at low temperature and this effect tended to decrease at elevated temperatures. When H2 was present in the feed, the kinetic model showed that H2 was consumed rapidly in the front part of the monolith. This was also seen in the experiments where in all cases H2 was entirely consumed. The rapid reaction of H2 along with resulting transport limitations indicated that the H2 promotion of the NO oxidation reaction may have been isolated to only a portion of the catalyst.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights• Microkinetic model of H2-assisted NO oxidation over Ag–Al2O3 monolith catalyst. • Single channel reactor model describes kinetics and mass transport. • Promoting effect of H2 proposed due to reduction of strongly adsorbed nitrate. • Rapid H2 consumption indicates H2 effect is isolated to a portion of catalyst.