| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 148513 | Chemical Engineering Journal | 2013 | 5 Pages |
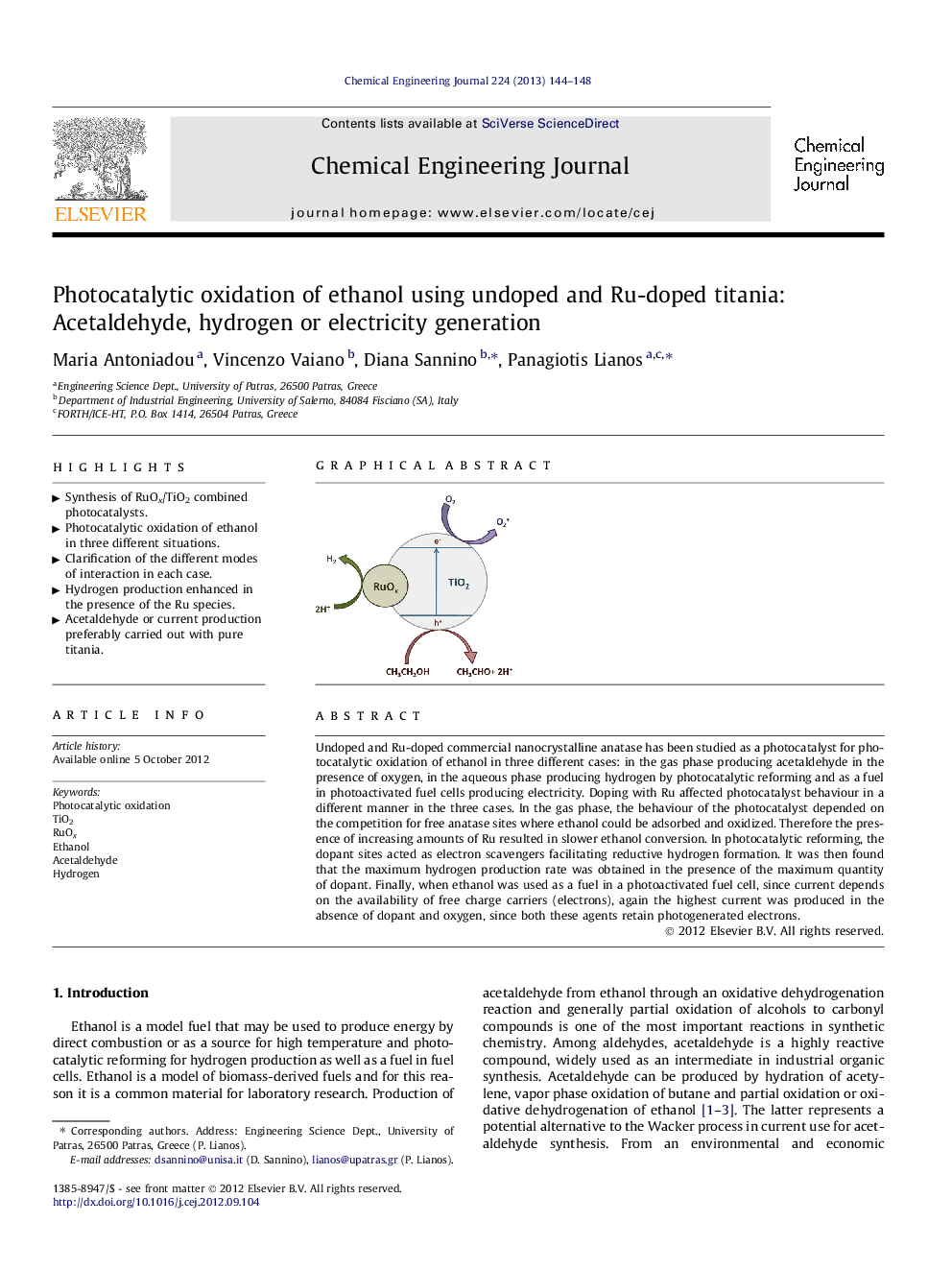
Undoped and Ru-doped commercial nanocrystalline anatase has been studied as a photocatalyst for photocatalytic oxidation of ethanol in three different cases: in the gas phase producing acetaldehyde in the presence of oxygen, in the aqueous phase producing hydrogen by photocatalytic reforming and as a fuel in photoactivated fuel cells producing electricity. Doping with Ru affected photocatalyst behaviour in a different manner in the three cases. In the gas phase, the behaviour of the photocatalyst depended on the competition for free anatase sites where ethanol could be adsorbed and oxidized. Therefore the presence of increasing amounts of Ru resulted in slower ethanol conversion. In photocatalytic reforming, the dopant sites acted as electron scavengers facilitating reductive hydrogen formation. It was then found that the maximum hydrogen production rate was obtained in the presence of the maximum quantity of dopant. Finally, when ethanol was used as a fuel in a photoactivated fuel cell, since current depends on the availability of free charge carriers (electrons), again the highest current was produced in the absence of dopant and oxygen, since both these agents retain photogenerated electrons.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Synthesis of RuOx/TiO2 combined photocatalysts. ► Photocatalytic oxidation of ethanol in three different situations. ► Clarification of the different modes of interaction in each case. ► Hydrogen production enhanced in the presence of the Ru species. ► Acetaldehyde or current production preferably carried out with pure titania.