| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 148552 | Chemical Engineering Journal | 2013 | 8 Pages |
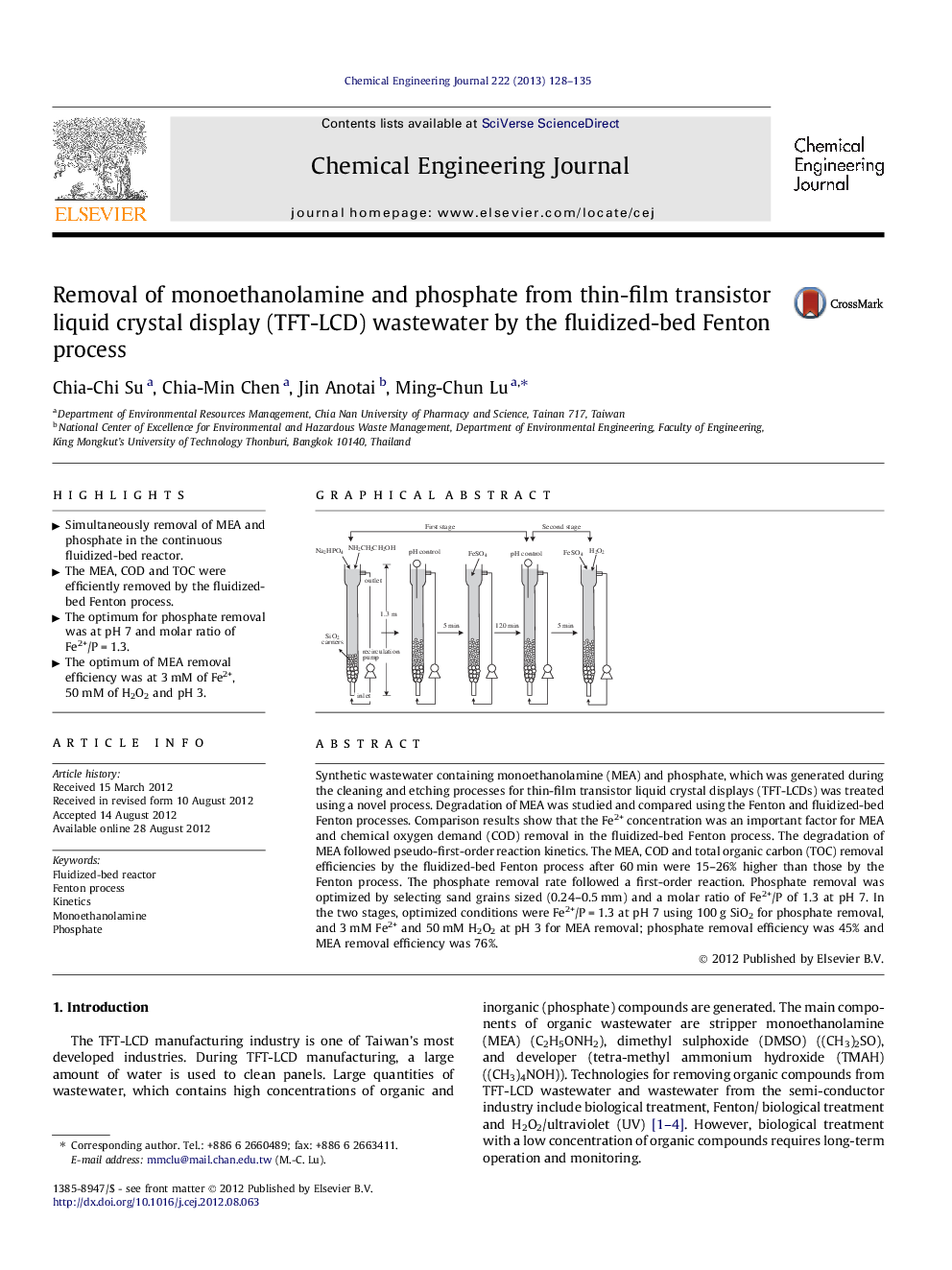
Synthetic wastewater containing monoethanolamine (MEA) and phosphate, which was generated during the cleaning and etching processes for thin-film transistor liquid crystal displays (TFT-LCDs) was treated using a novel process. Degradation of MEA was studied and compared using the Fenton and fluidized-bed Fenton processes. Comparison results show that the Fe2+ concentration was an important factor for MEA and chemical oxygen demand (COD) removal in the fluidized-bed Fenton process. The degradation of MEA followed pseudo-first-order reaction kinetics. The MEA, COD and total organic carbon (TOC) removal efficiencies by the fluidized-bed Fenton process after 60 min were 15–26% higher than those by the Fenton process. The phosphate removal rate followed a first-order reaction. Phosphate removal was optimized by selecting sand grains sized (0.24–0.5 mm) and a molar ratio of Fe2+/P of 1.3 at pH 7. In the two stages, optimized conditions were Fe2+/P = 1.3 at pH 7 using 100 g SiO2 for phosphate removal, and 3 mM Fe2+ and 50 mM H2O2 at pH 3 for MEA removal; phosphate removal efficiency was 45% and MEA removal efficiency was 76%.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Simultaneously removal of MEA and phosphate in the continuous fluidized-bed reactor. ► The MEA, COD and TOC were efficiently removed by the fluidized-bed Fenton process. ► The optimum for phosphate removal was at pH 7 and molar ratio of Fe2+/P = 1.3. ► The optimum of MEA removal efficiency was at 3 mM of Fe2+, 50 mM of H2O2 and pH 3.