| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 148555 | Chemical Engineering Journal | 2013 | 8 Pages |
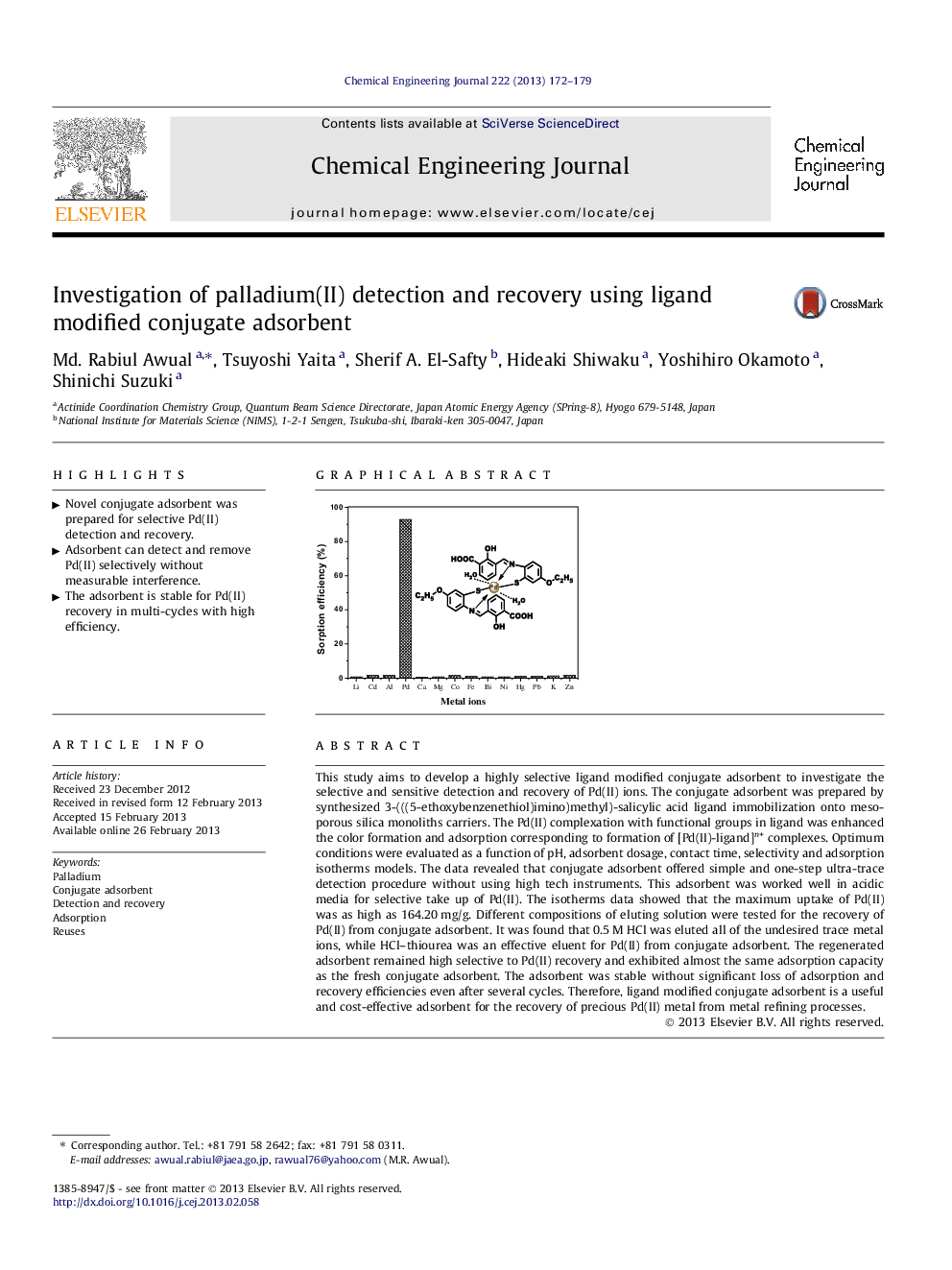
This study aims to develop a highly selective ligand modified conjugate adsorbent to investigate the selective and sensitive detection and recovery of Pd(II) ions. The conjugate adsorbent was prepared by synthesized 3-(((5-ethoxybenzenethiol)imino)methyl)-salicylic acid ligand immobilization onto mesoporous silica monoliths carriers. The Pd(II) complexation with functional groups in ligand was enhanced the color formation and adsorption corresponding to formation of [Pd(II)-ligand]n+ complexes. Optimum conditions were evaluated as a function of pH, adsorbent dosage, contact time, selectivity and adsorption isotherms models. The data revealed that conjugate adsorbent offered simple and one-step ultra-trace detection procedure without using high tech instruments. This adsorbent was worked well in acidic media for selective take up of Pd(II). The isotherms data showed that the maximum uptake of Pd(II) was as high as 164.20 mg/g. Different compositions of eluting solution were tested for the recovery of Pd(II) from conjugate adsorbent. It was found that 0.5 M HCl was eluted all of the undesired trace metal ions, while HCl–thiourea was an effective eluent for Pd(II) from conjugate adsorbent. The regenerated adsorbent remained high selective to Pd(II) recovery and exhibited almost the same adsorption capacity as the fresh conjugate adsorbent. The adsorbent was stable without significant loss of adsorption and recovery efficiencies even after several cycles. Therefore, ligand modified conjugate adsorbent is a useful and cost-effective adsorbent for the recovery of precious Pd(II) metal from metal refining processes.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Novel conjugate adsorbent was prepared for selective Pd(II) detection and recovery. ► Adsorbent can detect and remove Pd(II) selectively without measurable interference. ► The adsorbent is stable for Pd(II) recovery in multi-cycles with high efficiency.