| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 148618 | Chemical Engineering Journal | 2013 | 13 Pages |
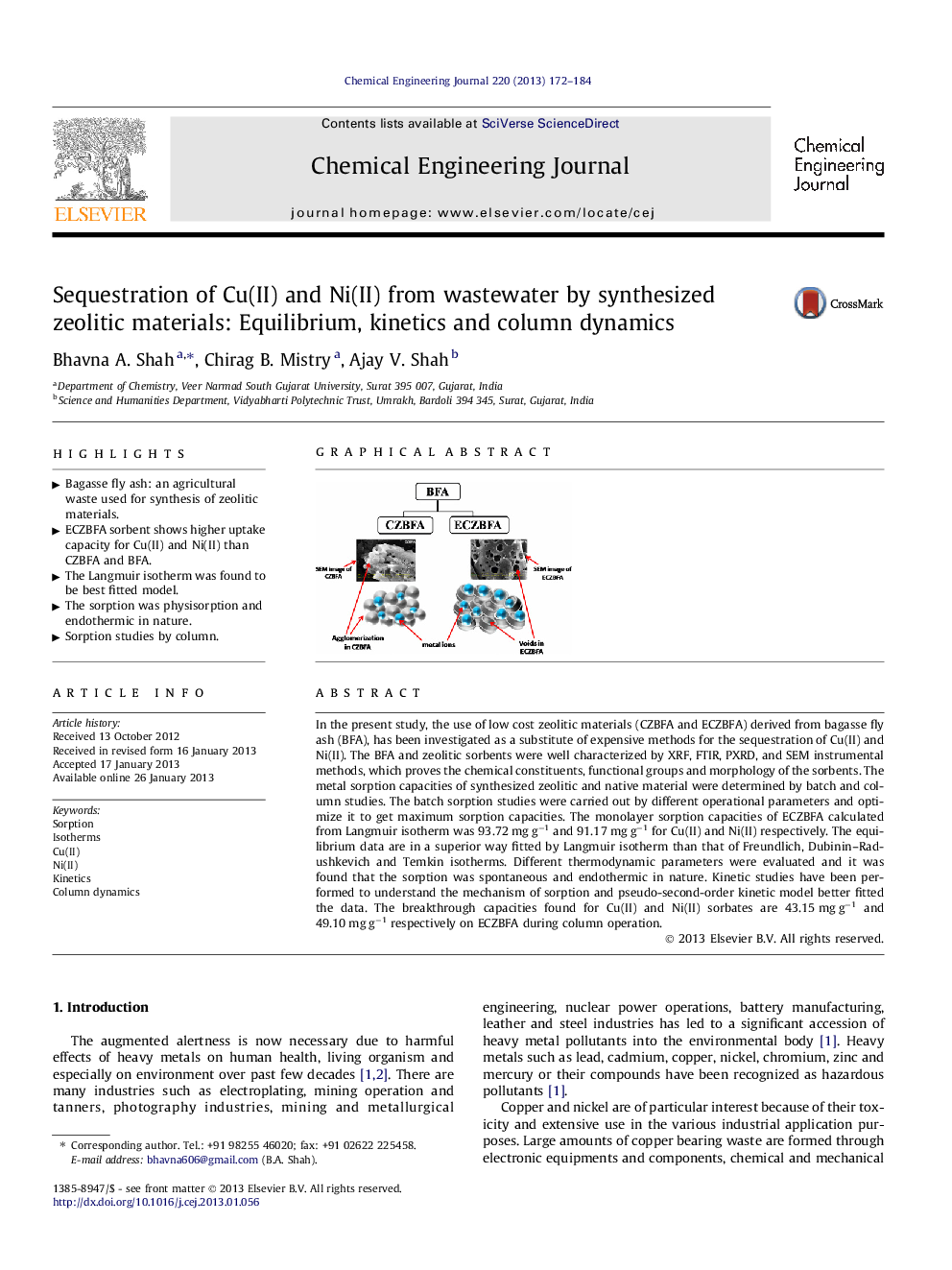
In the present study, the use of low cost zeolitic materials (CZBFA and ECZBFA) derived from bagasse fly ash (BFA), has been investigated as a substitute of expensive methods for the sequestration of Cu(II) and Ni(II). The BFA and zeolitic sorbents were well characterized by XRF, FTIR, PXRD, and SEM instrumental methods, which proves the chemical constituents, functional groups and morphology of the sorbents. The metal sorption capacities of synthesized zeolitic and native material were determined by batch and column studies. The batch sorption studies were carried out by different operational parameters and optimize it to get maximum sorption capacities. The monolayer sorption capacities of ECZBFA calculated from Langmuir isotherm was 93.72 mg g−1 and 91.17 mg g−1 for Cu(II) and Ni(II) respectively. The equilibrium data are in a superior way fitted by Langmuir isotherm than that of Freundlich, Dubinin–Radushkevich and Temkin isotherms. Different thermodynamic parameters were evaluated and it was found that the sorption was spontaneous and endothermic in nature. Kinetic studies have been performed to understand the mechanism of sorption and pseudo-second-order kinetic model better fitted the data. The breakthrough capacities found for Cu(II) and Ni(II) sorbates are 43.15 mg g−1 and 49.10 mg g−1 respectively on ECZBFA during column operation.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Bagasse fly ash: an agricultural waste used for synthesis of zeolitic materials. ► ECZBFA sorbent shows higher uptake capacity for Cu(II) and Ni(II) than CZBFA and BFA. ► The Langmuir isotherm was found to be best fitted model. ► The sorption was physisorption and endothermic in nature. ► Sorption studies by column.