| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 148660 | Chemical Engineering Journal | 2013 | 7 Pages |
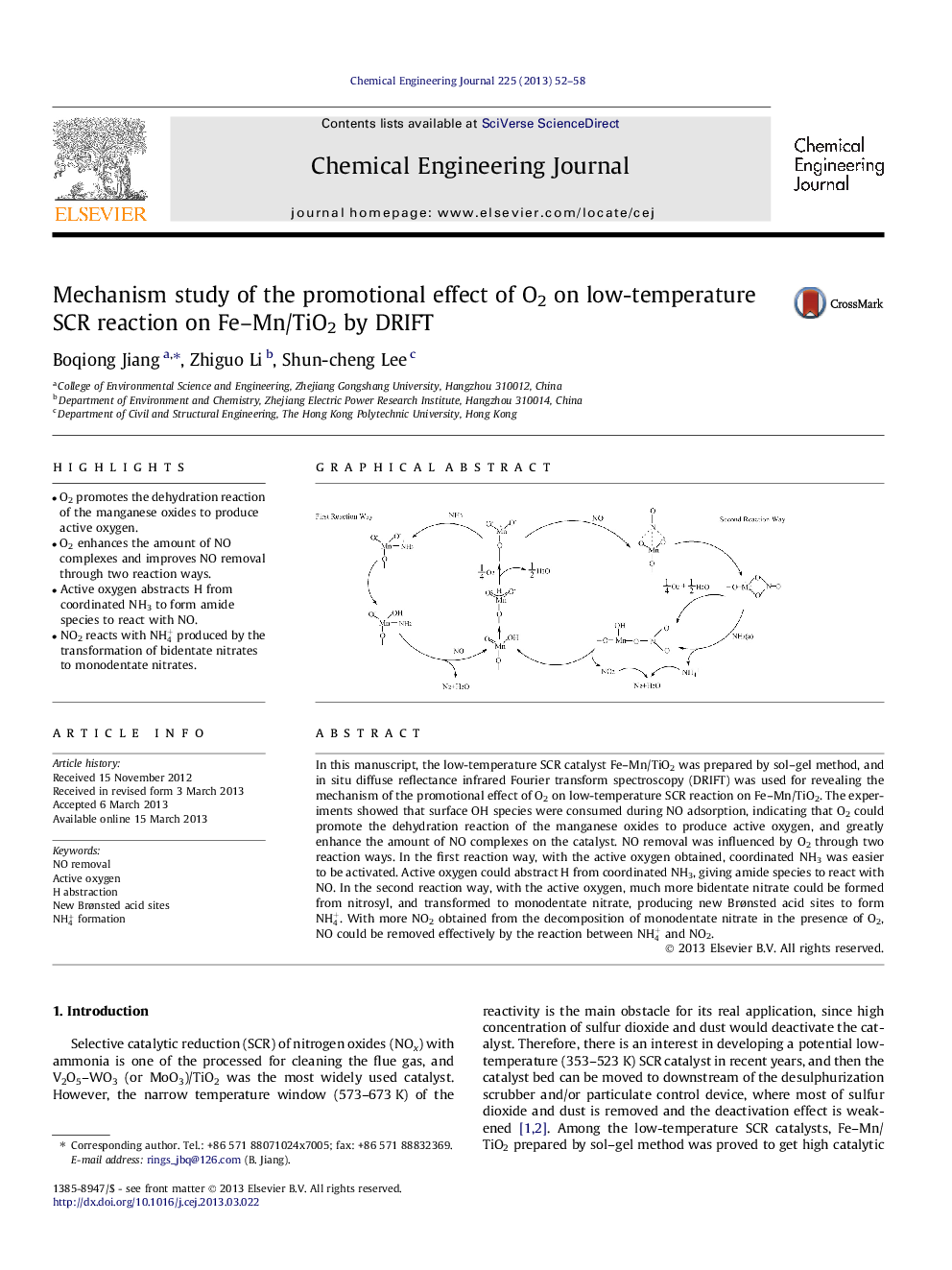
•O2 promotes the dehydration reaction of the manganese oxides to produce active oxygen.•O2 enhances the amount of NO complexes and improves NO removal through two reaction ways.•Active oxygen abstracts H from coordinated NH3 to form amide species to react with NO.•NO2 reacts with NH4+ produced by the transformation of bidentate nitrates to monodentate nitrates.
In this manuscript, the low-temperature SCR catalyst Fe–Mn/TiO2 was prepared by sol–gel method, and in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFT) was used for revealing the mechanism of the promotional effect of O2 on low-temperature SCR reaction on Fe–Mn/TiO2. The experiments showed that surface OH species were consumed during NO adsorption, indicating that O2 could promote the dehydration reaction of the manganese oxides to produce active oxygen, and greatly enhance the amount of NO complexes on the catalyst. NO removal was influenced by O2 through two reaction ways. In the first reaction way, with the active oxygen obtained, coordinated NH3 was easier to be activated. Active oxygen could abstract H from coordinated NH3, giving amide species to react with NO. In the second reaction way, with the active oxygen, much more bidentate nitrate could be formed from nitrosyl, and transformed to monodentate nitrate, producing new Brønsted acid sites to form NH4+. With more NO2 obtained from the decomposition of monodentate nitrate in the presence of O2, NO could be removed effectively by the reaction between NH4+ and NO2.
Graphical abstract.Figure optionsDownload full-size imageDownload as PowerPoint slide